p-cymene impairs SARS-CoV-2 and Influenza A (H1N1) viral replication: In silico predicted interaction with SARS-CoV-2 nucleocapsid protein and H1N1 nucleoprotein
- PMID: 34128351
- PMCID: PMC8204097
- DOI: 10.1002/prp2.798
p-cymene impairs SARS-CoV-2 and Influenza A (H1N1) viral replication: In silico predicted interaction with SARS-CoV-2 nucleocapsid protein and H1N1 nucleoprotein
Abstract
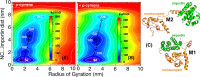
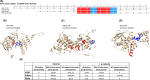
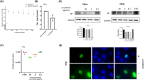
Therapeutic regimens for the COVID-19 pandemics remain unmet. In this line, repurposing of existing drugs against known or predicted SARS-CoV-2 protein actions have been advanced, while natural products have also been tested. Here, we propose that p-cymene, a natural monoterpene, can act as a potential novel agent for the treatment of SARS-CoV-2-induced COVID-19 and other RNA-virus-induced diseases (influenza, rabies, Ebola). We show by extensive molecular simulations that SARS-CoV-2 C-terminal structured domain contains a nuclear localization signal (NLS), like SARS-CoV, on which p-cymene binds with low micromolar affinity, impairing nuclear translocation of this protein and inhibiting viral replication, as verified by preliminary in vitro experiments. A similar mechanism may occur in other RNA-viruses (influenza, rabies and Ebola), also verified in vitro for influenza, by interaction of p-cymene with viral nucleoproteins, and structural modification of their NLS site, weakening its interaction with importin A. This common mechanism of action renders therefore p-cymene as a possible antiviral, alone, or in combination with other agents, in a broad spectrum of RNA viruses, from SARS-CoV-2 to influenza A infections.
Keywords: Ebola; SARS-CoV-2; importin A; influenza A; nucleocapsid protein; nucleoprotein; p-cymene; rabies.
© 2021 The Authors. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
AP, MT, CL, MK, SP, GS, and EC are inventors in a PCT patent application (priority # GR‐is 20200100068, GB‐2002141.6, PCT/EP2021/053389), related to the subject of this work. MK, GS, CL, SP, and EC are shareholders of the University of Crete spin‐off company Nature Crete Pharmaceuticals PC.
Figures


Similar articles
-
Glucosylceramide synthase inhibitors prevent replication of SARS-CoV-2 and influenza virus.J Biol Chem. 2021 Jan-Jun;296:100470. doi: 10.1016/j.jbc.2021.100470. Epub 2021 Feb 25. J Biol Chem. 2021. PMID: 33639165 Free PMC article.
-
Taurultam shows antiviral activity against SARS-CoV-2 and influenza virus.BMC Microbiol. 2025 May 15;25(1):292. doi: 10.1186/s12866-025-03847-2. BMC Microbiol. 2025. PMID: 40375181 Free PMC article.
-
Reduced Nucleoprotein Availability Impairs Negative-Sense RNA Virus Replication and Promotes Host Recognition.J Virol. 2021 Apr 12;95(9):e02274-20. doi: 10.1128/JVI.02274-20. Print 2021 Apr 12. J Virol. 2021. PMID: 33568513 Free PMC article.
-
How SARS-CoV-2 and Other Viruses Build an Invasion Route to Hijack the Host Nucleocytoplasmic Trafficking System.Cells. 2021 Jun 7;10(6):1424. doi: 10.3390/cells10061424. Cells. 2021. PMID: 34200500 Free PMC article. Review.
-
Antiviral Characterization of Advanced Materials: Use of Bacteriophage Phi 6 as Surrogate of Enveloped Viruses Such as SARS-CoV-2.Int J Mol Sci. 2022 May 10;23(10):5335. doi: 10.3390/ijms23105335. Int J Mol Sci. 2022. PMID: 35628148 Free PMC article. Review.
Cited by
-
Discovery of a new generation of angiotensin receptor blocking drugs: Receptor mechanisms and in silico binding to enzymes relevant to SARS-CoV-2.Comput Struct Biotechnol J. 2022;20:2091-2111. doi: 10.1016/j.csbj.2022.04.010. Epub 2022 Apr 9. Comput Struct Biotechnol J. 2022. PMID: 35432786 Free PMC article.
-
From Traditional Ethnopharmacology to Modern Natural Drug Discovery: A Methodology Discussion and Specific Examples.Molecules. 2022 Jun 24;27(13):4060. doi: 10.3390/molecules27134060. Molecules. 2022. PMID: 35807306 Free PMC article. Review.
-
Computational exploration of the dual role of the phytochemical fortunellin: Antiviral activities against SARS-CoV-2 and immunomodulatory abilities against the host.Comput Biol Med. 2022 Oct;149:106049. doi: 10.1016/j.compbiomed.2022.106049. Epub 2022 Sep 8. Comput Biol Med. 2022. PMID: 36103744 Free PMC article.
-
Insights into the specificity for the interaction of the promiscuous SARS-CoV-2 nucleocapsid protein N-terminal domain with deoxyribonucleic acids.Int J Biol Macromol. 2022 Apr 1;203:466-480. doi: 10.1016/j.ijbiomac.2022.01.121. Epub 2022 Jan 22. Int J Biol Macromol. 2022. PMID: 35077748 Free PMC article.
-
Proliferative Effect of Aqueous Extract of Sea Cucumber (Holothuria parva) Body Wall on Human Umbilical Cord Mesenchymal Stromal/Stem Cells.Mar Drugs. 2023 Apr 26;21(5):267. doi: 10.3390/md21050267. Mar Drugs. 2023. PMID: 37233461 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

