Carrageenan nasal spray may double the rate of recovery from coronavirus and influenza virus infections: Re-analysis of randomized trial data
- PMID: 34128358
- PMCID: PMC8204093
- DOI: 10.1002/prp2.810
Carrageenan nasal spray may double the rate of recovery from coronavirus and influenza virus infections: Re-analysis of randomized trial data
Abstract
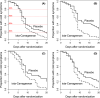
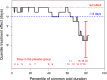
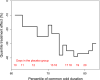
In this individual patient data meta-analysis we examined datasets of two randomized placebo-controlled trials which investigated the effect of nasal carrageenan separately on children and adults. In both trials, iota-carrageenan was administered nasally three times per day for 7 days for patients with the common cold and follow-up lasted for 21 days. We used Cox regression to estimate the effect of carrageenan on recovery rate. We also used quantile regression to calculate the effect of carrageenan on colds of differing lengths. Nasal carrageenan increased the recovery rate from all colds by 54% (95% CI 15%-105%; p = .003). The increase in recovery rate was 139% for coronavirus infections, 119% for influenza A infections, and 70% for rhinovirus infections. The mean duration of all colds in the placebo groups of the first four quintiles were 4.0, 6.8, 8.8, and 13.7 days, respectively. The fifth quintile contained patients with censored data. The 13.7-day colds were shortened by 3.8 days (28% reduction), and 8.8-day colds by 1.3 days (15% reduction). Carrageenan had no meaningful effect on shorter colds. In the placebo group, 21 patients had colds lasting over 20 days, compared with six patients in the carrageenan group, which corresponds to a 71% (p = .003) reduction in the risk of longer colds. Given that carrageenan has an effect on diverse virus groups, and effects at the clinical level on two old coronaviruses, it seems plausible that carrageenan may have an effect on COVID-19. Further research on nasal iota-carrageenan is warranted.
Keywords: SARS-CoV-2; common cold; iota-carrageenan; meta-analysis; quantile treatment effect; randomized trial; rhinovirus.
© 2021 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial.Respir Res. 2013 Nov 13;14(1):124. doi: 10.1186/1465-9921-14-124. Respir Res. 2013. PMID: 24219370 Free PMC article. Clinical Trial.
-
Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial.Respir Res. 2015 Oct 5;16:121. doi: 10.1186/s12931-015-0281-8. Respir Res. 2015. PMID: 26438038 Free PMC article. Clinical Trial.
-
Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold.BMC Complement Altern Med. 2012 Sep 5;12:147. doi: 10.1186/1472-6882-12-147. BMC Complement Altern Med. 2012. PMID: 22950667 Free PMC article. Clinical Trial.
-
The antiviral activity of iota-, kappa-, and lambda-carrageenan against COVID-19: A critical review.Clin Epidemiol Glob Health. 2021 Oct-Dec;12:100826. doi: 10.1016/j.cegh.2021.100826. Epub 2021 Jun 29. Clin Epidemiol Glob Health. 2021. PMID: 34222718 Free PMC article. Review.
-
Clinical applications of antiviral agents for chemophrophylaxis and therapy of respiratory viral infections.Antiviral Res. 1985;Suppl 1:229-39. doi: 10.1016/s0166-3542(85)80033-4. Antiviral Res. 1985. PMID: 2417551 Review.
Cited by
-
A Supine Position and Dual-Dose Applications Enhance Spray Dosing to the Posterior Nose: Paving the Way for Mucosal Immunization.Pharmaceutics. 2023 Jan 20;15(2):359. doi: 10.3390/pharmaceutics15020359. Pharmaceutics. 2023. PMID: 36839681 Free PMC article.
-
Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease.Int J Gen Med. 2021 Oct 1;14:6277-6286. doi: 10.2147/IJGM.S328486. eCollection 2021. Int J Gen Med. 2021. PMID: 34629893 Free PMC article. Clinical Trial.
-
Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia.Biochem Biophys Rep. 2022 Mar;29:101187. doi: 10.1016/j.bbrep.2021.101187. Epub 2021 Dec 15. Biochem Biophys Rep. 2022. PMID: 34931176 Free PMC article.
-
Antiviral Strategies Using Natural Source-Derived Sulfated Polysaccharides in the Light of the COVID-19 Pandemic and Major Human Pathogenic Viruses.Viruses. 2021 Dec 24;14(1):35. doi: 10.3390/v14010035. Viruses. 2021. PMID: 35062238 Free PMC article. Review.
-
Marine Sulfated Polysaccharides as Promising Antiviral Agents: A Comprehensive Report and Modeling Study Focusing on SARS CoV-2.Mar Drugs. 2021 Jul 22;19(8):406. doi: 10.3390/md19080406. Mar Drugs. 2021. PMID: 34436245 Free PMC article.
References
-
- Eccles R. Iota‐carrageenan as an antiviral treatment for the common cold. Open Virol J. 2020;14:9‐15. 10.2174/1874357902014010009 - DOI
-
- González ME, Alarcón B, Carrasco L. Polysaccharides as antiviral agents: antiviral activity of carrageenan. Antimicrob Agents Chemother. 1987;31(9):1388‐1393. 10.1128/aac.31.9.1388. http://www.ncbi.nlm.nih.gov/pmc/articles/pmc174948/ - DOI - PMC - PubMed
-
- Baba M, Snoeck R, Pauwels R, de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988;32(11):1742‐1745. 10.1128/aac.32.11.1742. http://www.ncbi.nlm.nih.gov/pmc/articles/pmc175964/ - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

