Studying human nociceptors: from fundamentals to clinic
- PMID: 34128530
- PMCID: PMC8219361
- DOI: 10.1093/brain/awab048
Studying human nociceptors: from fundamentals to clinic
Abstract
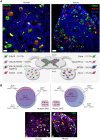
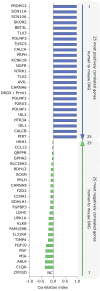
Chronic pain affects one in five of the general population and is the third most important cause of disability-adjusted life-years globally. Unfortunately, treatment remains inadequate due to poor efficacy and tolerability. There has been a failure in translating promising preclinical drug targets into clinic use. This reflects challenges across the whole drug development pathway, from preclinical models to trial design. Nociceptors remain an attractive therapeutic target: their sensitization makes an important contribution to many chronic pain states, they are located outside the blood-brain barrier, and they are relatively specific. The past decade has seen significant advances in the techniques available to study human nociceptors, including: the use of corneal confocal microscopy and biopsy samples to observe nociceptor morphology, the culture of human nociceptors (either from surgical or post-mortem tissue or using human induced pluripotent stem cell derived nociceptors), the application of high throughput technologies such as transcriptomics, the in vitro and in vivo electrophysiological characterization through microneurography, and the correlation with pain percepts provided by quantitative sensory testing. Genome editing in human induced pluripotent stem cell-derived nociceptors enables the interrogation of the causal role of genes in the regulation of nociceptor function. Both human and rodent nociceptors are more heterogeneous at a molecular level than previously appreciated, and while we find that there are broad similarities between human and rodent nociceptors there are also important differences involving ion channel function, expression, and cellular excitability. These technological advances have emphasized the maladaptive plastic changes occurring in human nociceptors following injury that contribute to chronic pain. Studying human nociceptors has revealed new therapeutic targets for the suppression of chronic pain and enhanced repair. Cellular models of human nociceptors have enabled the screening of small molecule and gene therapy approaches on nociceptor function, and in some cases have enabled correlation with clinical outcomes. Undoubtedly, challenges remain. Many of these techniques are difficult to implement at scale, current induced pluripotent stem cell differentiation protocols do not generate the full diversity of nociceptor populations, and we still have a relatively poor understanding of inter-individual variation in nociceptors due to factors such as age, sex, or ethnicity. We hope our ability to directly investigate human nociceptors will not only aid our understanding of the fundamental neurobiology underlying acute and chronic pain but also help bridge the translational gap.
Keywords: IPSC derived nociceptors; microneurography; nociceptors; patch-clamp; transcriptomics.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



References
-
- Sherrington CS. The integrative action of the nervous system. C. Scribner's sons; 1906.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

