Developing a Framework for Pandemic COVID-19 Vaccine Allocation: a Modified Delphi Consensus Study in Korea
- PMID: 34128597
- PMCID: PMC8203851
- DOI: 10.3346/jkms.2021.36.e166
Developing a Framework for Pandemic COVID-19 Vaccine Allocation: a Modified Delphi Consensus Study in Korea
Abstract
Background: This study presents a framework for determining the allocation and distribution of the limited amount of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
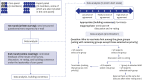
Methods: After analyzing the pandemic strategies of the major organizations and countries and with a literature review conducted by a core panel, a modified Delphi survey was administered to 13 experts in the fields of vaccination, infectious disease, and public health in the Republic of Korea. The following topics were discussed: 1) identifying the objectives of the vaccination strategy, 2) identifying allocation criteria, and 3) establishing a step-by-step vaccination framework and prioritization strategy based on the allocation criteria. Two rounds of surveys were conducted for each topic, with a structured questionnaire provided via e-mail in the first round. After analyzing the responses, a meeting with the experts was held to obtain consensus on how to prioritize the population groups.
Results: The first objective of the vaccination strategy was maintenance of the integrity of the healthcare system and critical infrastructure, followed by reduction of morbidity and mortality and reduction of community transmission. In the initial phase, older adult residents in care homes, high-risk health and social care workers, and personal support workers who work in direct contact with coronavirus disease 2019 (COVID-19) patients would be prioritized. Expansion of vaccine supply would allow immunization of older adults not included in phase 1, followed by healthcare workers not previously included and individuals with comorbidities. Further widespread vaccine supply would ensure availability to the extended adult age groups (50-64 years old), critical workers outside the health sector, residents who cannot socially distance, and, eventually, the remaining populations.
Conclusion: This survey provides the much needed insight into the decision-making process for vaccine allocation at the national level. However, flexibility in adapting to strategies will be essential, as new information is constantly emerging.
Keywords: COVID-19; Distribution; Korea; Policy; Survey; Vaccines.
© 2021 The Korean Academy of Medical Sciences.
Conflict of interest statement
The opinions expressed by authors contributing to this article do not necessarily reflect the opinions of the Korea Disease Control and Prevention Agency or the institutions with which the authors are affiliated. The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Who should be prioritized for COVID-19 vaccination in China? A descriptive study.BMC Med. 2021 Feb 10;19(1):45. doi: 10.1186/s12916-021-01923-8. BMC Med. 2021. PMID: 33563270 Free PMC article.
-
Public Perspectives on COVID-19 Vaccine Prioritization.JAMA Netw Open. 2021 Apr 1;4(4):e217943. doi: 10.1001/jamanetworkopen.2021.7943. JAMA Netw Open. 2021. PMID: 33835172 Free PMC article.
-
Dynamics of the COVID-19 epidemic in the post-vaccination period in Korea: a rapid assessment.Epidemiol Health. 2021;43:e2021040. doi: 10.4178/epih.e2021040. Epub 2021 May 27. Epidemiol Health. 2021. PMID: 34082498 Free PMC article.
-
Is there a role for childhood vaccination against COVID-19?Pediatr Allergy Immunol. 2021 Jan;32(1):9-16. doi: 10.1111/pai.13401. Epub 2020 Nov 20. Pediatr Allergy Immunol. 2021. PMID: 33113210 Review.
-
[Consensus of the Genetics Branch of the Chilean Society of Pediatrics on the prioritization of people with Down syndrome and rare diseases for vaccination against SARS-CoV-2].Andes Pediatr. 2021 Apr;92(2):309-315. doi: 10.32641/andespediatr.v92i2.3716. Andes Pediatr. 2021. PMID: 34106172 Spanish.
Cited by
-
Short Term Impact of Coronavirus Disease 2019 Vaccination in Children in Korea.J Korean Med Sci. 2022 May 2;37(17):e124. doi: 10.3346/jkms.2022.37.e124. J Korean Med Sci. 2022. PMID: 35502499 Free PMC article.
-
Non-EPI Vaccine Hesitancy among Chinese Adults: A Cross-Sectional Study.Vaccines (Basel). 2021 Jul 10;9(7):772. doi: 10.3390/vaccines9070772. Vaccines (Basel). 2021. PMID: 34358188 Free PMC article.
-
Age discrimination, the right to life, and COVID-19 vaccination in countries with limited resources.J Soc Issues. 2022 Sep 30:10.1111/josi.12561. doi: 10.1111/josi.12561. Online ahead of print. J Soc Issues. 2022. PMID: 36249552 Free PMC article.
-
Impact of national Covid-19 vaccination Campaign, South Korea.Vaccine. 2022 Jun 9;40(26):3670-3675. doi: 10.1016/j.vaccine.2022.05.002. Epub 2022 May 8. Vaccine. 2022. PMID: 35570077 Free PMC article.
-
Adult Immunization Policy in Korea.Infect Chemother. 2023 Sep;55(3):317-321. doi: 10.3947/ic.2023.0089. Infect Chemother. 2023. PMID: 37794577 Free PMC article. Review.
References
-
- World Health Organization. Weekly epidemiological update on COVID-19 - 6 April 2021. [Updated April 4, 2021]. [Accessed April 6, 2021]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on....
-
- European Centre for Disease Prevention and Control. Rapid risk assessment: coronavirus disease 2019 (COVID-19) in the EU/EEA and the UK – eighth update. 8 April 2020. [Updated April 8, 2020]. [Accessed November 29, 2020]. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-rapid-....
-
- Centers for Disease Control and Prevention. COVID data tracker. Nationwide commercial laboratory seroprevalence survey. [Accessed March 2, 2021]. https://covid.cdc.gov/covid-data-tracker/#national-lab.
-
- European Centre for Disease Prevention and Control. Immune responses and immunity to SARS-CoV-2. [Accessed November 29, 2020]. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous