Tethered Silanoxyiodination of Alkenes
- PMID: 34128664
- PMCID: PMC9012987
- DOI: 10.1021/acs.joc.1c00872
Tethered Silanoxyiodination of Alkenes
Abstract
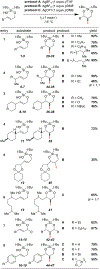
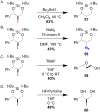
We present the first examples of tethered silanoxyiodination reactions of allylic alcohols. The products are useful silanediol organoiodide synthons and are formed with high regioselectivity and diastereocontrol. The reaction is scalable greater than 10-fold without loss of yield or selectivity. Furthermore, the products are starting materials for further transformations, including deiodination, C-N bond installation, epoxide synthesis, and desilylation. DFT calculations provide a basis for understanding the exquisite 6-endo selectivity of this silanoxyiodination reaction and show that the observed products are both kinetically and thermodynamically preferred.
Figures



References
-
- Degennaro L; Trinchera P; Luisi R, Recent Advances in the Stereoselective Synthesis of Aziridines. Chem. Rev 2014, 114, 7881–7929. - PubMed
-
- Overman LE; Campbell CB, Hemiacetal mediated reactions. Directed synthesis of diols and acetals. J. Org. Chem 1974, 39, 1474–1481.
-
- Sarraf ST; Leighton JL, Oxymercuration of Homoallylic Alcohol Derived Hemiacetals: Diastereoselective Synthesis of Protected 1,3-Diols. Org. Lett 2000, 2, 403–405. - PubMed
-
- Kotov V; Scarborough CC; Stahl SS, Palladium-Catalyzed Aerobic Oxidative Amination of Alkenes: Development of Intra- and Intermolecular Aza-Wacker Reactions. Inorg. Chem 2007, 46, 1910–1923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

