Dynamic innate immune response determines susceptibility to SARS-CoV-2 infection and early replication kinetics
- PMID: 34128960
- PMCID: PMC8210587
- DOI: 10.1084/jem.20210583
Dynamic innate immune response determines susceptibility to SARS-CoV-2 infection and early replication kinetics
Abstract
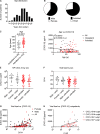
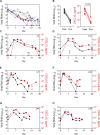
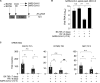
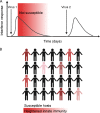
Initial replication of SARS-CoV-2 in the upper respiratory tract is required to establish infection, and the replication level correlates with the likelihood of viral transmission. Here, we examined the role of host innate immune defenses in restricting early SARS-CoV-2 infection using transcriptomics and biomarker-based tracking in serial patient nasopharyngeal samples and experiments with airway epithelial organoids. SARS-CoV-2 initially replicated exponentially, with a doubling time of ∼6 h, and induced interferon-stimulated genes (ISGs) in the upper respiratory tract, which rose with viral replication and peaked just as viral load began to decline. Rhinovirus infection before SARS-CoV-2 exposure accelerated ISG responses and prevented SARS-CoV-2 replication. Conversely, blocking ISG induction during SARS-CoV-2 infection enhanced viral replication from a low infectious dose. These results show that the activity of ISG-mediated defenses at the time of SARS-CoV-2 exposure impacts infection progression and that the heterologous antiviral response induced by a different virus can protect against SARS-CoV-2.
© 2021 Cheemarla et al.
Conflict of interest statement
Disclosures: E.F. Foxman reported a patent application to WO2019/217296 A1 pending, and E.F. Foxman and M. L. Landry reported a patent application to WO2018/071498 pending. No other disclosures were reported.
Figures









Update of
-
Magnitude and timing of the antiviral response determine SARS-CoV-2 replication early in infection.medRxiv [Preprint]. 2021 Jan 27:2021.01.22.21249812. doi: 10.1101/2021.01.22.21249812. medRxiv. 2021. Update in: J Exp Med. 2021 Aug 2;218(8):e20210583. doi: 10.1084/jem.20210583. PMID: 33532783 Free PMC article. Updated. Preprint.
Comment in
-
The early interferon catches the SARS-CoV-2.J Exp Med. 2021 Oct 4;218(10):e20211667. doi: 10.1084/jem.20211667. Epub 2021 Aug 23. J Exp Med. 2021. PMID: 34424267 Free PMC article.
References
-
- Asgari, S., Schlapbach L.J., Anchisi S., Hammer C., Bartha I., Junier T., Mottet-Osman G., Posfay-Barbe K.M., Longchamp D., Stocker M., et al. . 2017. Severe viral respiratory infections in children with IFIH1 loss-of-function mutations. Proc. Natl. Acad. Sci. USA. 114:8342–8347. 10.1073/pnas.1704259114 - DOI - PMC - PubMed
-
- Banerjee, A.K., Blanco M.R., Bruce E.A., Honson D.D., Chen L.M., Chow A., Bhat P., Ollikainen N., Quinodoz S.A., Loney C., et al. . 2020a. SARS-CoV-2 Disrupts Splicing, Translation, and Protein Trafficking to Suppress Host Defenses. Cell. 183:1325–1339.e21. 10.1016/j.cell.2020.10.004 - DOI - PMC - PubMed
-
- Banerjee, A.K., Blanco M.R., Bruce E.A., Honson D.D., Chen L.M., Chow A., Bhat P., Ollikainen N., Quinodoz S.A., Loney C., et al. . 2020b. SARS-CoV-2 Disrupts Splicing, Translation, and Protein Trafficking to Suppress Host Defenses. Cell. 183:1325–1339.e21. 10.1016/j.cell.2020.10.004 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

