Induced pluripotent stem cell-derived motor neurons of CMT type 2 patients reveal progressive mitochondrial dysfunction
- PMID: 34128983
- PMCID: PMC8418338
- DOI: 10.1093/brain/awab226
Induced pluripotent stem cell-derived motor neurons of CMT type 2 patients reveal progressive mitochondrial dysfunction
Abstract
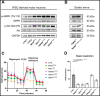
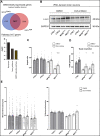
Axonal Charcot-Marie-Tooth neuropathies (CMT type 2) are caused by inherited mutations in various genes functioning in different pathways. The types of genes and multiplicity of mutations reflect the clinical and genetic heterogeneity in CMT2 disease, which complicates its diagnosis and has inhibited the development of therapies. Here, we used CMT2 patient-derived pluripotent stem cells (iPSCs) to identify common hallmarks of axonal degeneration shared by different CMT2 subtypes. We compared the cellular phenotypes of neurons differentiated from CMT2 patient iPSCs with those from healthy controls and a CRISPR/Cas9-corrected isogenic line. Our results demonstrated neurite network alterations along with extracellular electrophysiological abnormalities in the differentiated motor neurons. Progressive deficits in mitochondrial and lysosomal trafficking, as well as in mitochondrial morphology, were observed in all CMT2 patient lines. Differentiation of the same CMT2 iPSC lines into peripheral sensory neurons only gave rise to cellular phenotypes in subtypes with sensory involvement, supporting the notion that some gene mutations predominantly affect motor neurons. We revealed a common mitochondrial dysfunction in CMT2-derived motor neurons, supported by alterations in the expression pattern and oxidative phosphorylation, which could be recapitulated in the sciatic nerve tissue of a symptomatic mouse model. Inhibition of a dual leucine zipper kinase could partially ameliorate the mitochondrial disease phenotypes in CMT2 subtypes. Altogether, our data reveal shared cellular phenotypes across different CMT2 subtypes and suggests that targeting such common pathomechanisms could allow the development of a uniform treatment for CMT2.
Keywords: Charcot-Marie-Tooth neuropathy; dual leucine kinase inhibitor; iPSC-derived motor and sensory neurons; mitochondrial dysfunction; phenotyping.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
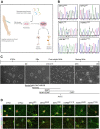
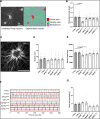
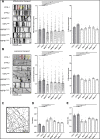
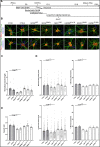

Comment in
-
Modelling Charcot-Marie-Tooth disease in a dish reveals common cell type-specific alterations.Brain. 2021 Sep 4;144(8):2234-2236. doi: 10.1093/brain/awab278. Brain. 2021. PMID: 34318312 Free PMC article.
References
-
- Baets J, De Jonghe P, Timmerman V.. Recent advances in Charcot-Marie-Tooth disease. Curr Opin Neurol. 2014;27(5):532–540. - PubMed
-
- Pisciotta C, Shy ME.. Neuropathy. Handbook Clin Neurol. 2018;148:653–665. - PubMed
-
- Pareyson D.Axonal Charcot-Marie-Tooth disease: The fog is only slowly lifting. Neurology. 2007;68(20):1649–1650. - PubMed
-
- Pipis M, Rossor AM, Laura M, Reilly MM.. Next-generation sequencing in Charcot–Marie–Tooth disease: Opportunities and challenges. Nat Rev Neurol. 2019;15(11):644–656. - PubMed
-
- Takahashi K, Yamanaka S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

