Effects of Intravenous Eptinezumab vs Placebo on Headache Pain and Most Bothersome Symptom When Initiated During a Migraine Attack: A Randomized Clinical Trial
- PMID: 34128999
- PMCID: PMC8207242
- DOI: 10.1001/jama.2021.7665
Effects of Intravenous Eptinezumab vs Placebo on Headache Pain and Most Bothersome Symptom When Initiated During a Migraine Attack: A Randomized Clinical Trial
Abstract
Importance: Intravenous eptinezumab, an anti-calcitonin gene-related peptide antibody, is approved for migraine prevention in adults. It has established onset of preventive efficacy on day 1 after infusion.
Objective: To evaluate the efficacy of and adverse events related to eptinezumab when initiated during a migraine attack.
Design, setting, and participants: Phase 3, multicenter, parallel-group, double-blind, randomized, placebo-controlled trial conducted from November 4, 2019, to July 8, 2020, at 47 sites in the United States and the country of Georgia. Participants (aged 18-75 years) with a greater than 1-year history of migraine and migraine on 4 to 15 days per month in the 3 months prior to screening were treated during a moderate to severe migraine attack.
Interventions: Eptinezumab, 100 mg (n = 238), or placebo (n = 242), administered intravenously within 1 to 6 hours of onset of a qualifying moderate to severe migraine.
Main outcomes and measures: Co-primary efficacy end points were time to headache pain freedom and time to absence of most bothersome symptom (nausea, photophobia, or phonophobia). Key secondary end points were headache pain freedom and absence of most bothersome symptom at 2 hours after start of infusion. Additional secondary end points were headache pain freedom and absence of most bothersome symptom at 4 hours and use of rescue medication within 24 hours.
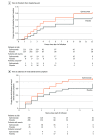
Results: Of 480 randomized and treated patients (mean age, 44 years; 84% female), 476 completed the study. Patients treated with eptinezumab vs placebo, respectively, achieved statistically significantly faster headache pain freedom (median, 4 hours vs 9 hours; hazard ratio, 1.54 [P < .001]) and absence of most bothersome symptom (median, 2 hours vs 3 hours; hazard ratio, 1.75 [P < .001]). At 2 hours after infusion, in the respective eptinezumab and placebo groups, headache pain freedom was achieved by 23.5% and 12.0% (between-group difference, 11.6% [95% CI, 4.78%-18.31%]; odds ratio, 2.27 [95% CI, 1.39-3.72]; P < .001) and absence of most bothersome symptom by 55.5% and 35.8% (between-group difference, 19.6% [95% CI, 10.87%-28.39%]; odds ratio, 2.25 [95% CI, 1.55-3.25]; P < .001). Results remained statistically significant at 4 hours after infusion. Statistically significantly fewer eptinezumab-treated patients used rescue medication within 24 hours than did placebo patients (31.5% vs 59.9%, respectively; between-group difference, -28.4% [95% CI, -36.95% to -19.86%]; odds ratio, 0.31 [95% CI, 0.21-0.45]; P < .001). Treatment-emergent adverse events occurred in 10.9% of the eptinezumab group and 10.3% of the placebo group; the most common was hypersensitivity (eptinezumab, 2.1%; placebo, 0%). No treatment-emergent serious adverse events occurred.
Conclusions and relevance: Among patients eligible for preventive migraine therapy experiencing a moderate to severe migraine attack, treatment with intravenous eptinezumab vs placebo shortened time to headache and symptom resolution. Feasibility of administering eptinezumab treatment during a migraine attack and comparison with alternative treatments remain to be established.
Trial registration: ClinicalTrials.gov Identifier: NCT04152083.
Conflict of interest statement
Figures

Comment in
-
Acute Treatment for Migraine: Contemporary Treatments and Future Directions.JAMA. 2021 Jun 15;325(23):2346-2347. doi: 10.1001/jama.2021.7275. JAMA. 2021. PMID: 34129013 No abstract available.
Similar articles
-
Rapid resolution of migraine symptoms after initiating the preventive treatment eptinezumab during a migraine attack: results from the randomized RELIEF trial.BMC Neurol. 2022 Jun 3;22(1):205. doi: 10.1186/s12883-022-02714-1. BMC Neurol. 2022. PMID: 35659622 Free PMC article. Clinical Trial.
-
Eptinezumab treatment initiated during a migraine attack is associated with meaningful improvement in patient-reported outcome measures: secondary results from the randomized controlled RELIEF study.J Headache Pain. 2022 Feb 7;23(1):22. doi: 10.1186/s10194-021-01376-7. J Headache Pain. 2022. PMID: 35130832 Free PMC article. Clinical Trial.
-
Effect of Ubrogepant vs Placebo on Pain and the Most Bothersome Associated Symptom in the Acute Treatment of Migraine: The ACHIEVE II Randomized Clinical Trial.JAMA. 2019 Nov 19;322(19):1887-1898. doi: 10.1001/jama.2019.16711. JAMA. 2019. PMID: 31742631 Free PMC article. Clinical Trial.
-
Efficacy and safety of eptinezumab as preventive treatment for episodic/chronic migraine: A systematic review and meta-analysis.Clin Exp Pharmacol Physiol. 2022 Nov;49(11):1156-1168. doi: 10.1111/1440-1681.13700. Epub 2022 Jul 20. Clin Exp Pharmacol Physiol. 2022. PMID: 35781694
-
Safety, efficacy, and compliance of moderate-to-high dose eptinezumab and erenumab in chronic migraine patients with medication-overuse headache: an updated systematic review and meta-analysis.J Headache Pain. 2025 May 6;26(1):99. doi: 10.1186/s10194-025-02047-7. J Headache Pain. 2025. PMID: 40329188 Free PMC article.
Cited by
-
The Arrival of Anti-CGRP Monoclonal Antibodies in Migraine.Neurotherapeutics. 2022 Apr;19(3):922-930. doi: 10.1007/s13311-022-01230-x. Epub 2022 Apr 14. Neurotherapeutics. 2022. PMID: 35426060 Free PMC article. Review.
-
Eptinezumab for migraine.Aust Prescr. 2022 Jun;45(3):97-98. doi: 10.18773/austprescr.2022.030. Epub 2022 Apr 29. Aust Prescr. 2022. PMID: 35755991 Free PMC article. Review. No abstract available.
-
Eptinezumab for the preventive treatment of episodic and chronic migraine: a narrative review.Front Neurol. 2024 Mar 8;15:1355877. doi: 10.3389/fneur.2024.1355877. eCollection 2024. Front Neurol. 2024. PMID: 38523607 Free PMC article. Review.
-
Adverse and serious adverse events incidence of pharmacological interventions for managing chronic and episodic migraine in adults: a systematic review.BMJ Neurol Open. 2024 Apr 17;6(1):e000616. doi: 10.1136/bmjno-2023-000616. eCollection 2024. BMJ Neurol Open. 2024. PMID: 38646505 Free PMC article.
-
Preventive drug treatments for adults with chronic migraine: a systematic review with economic modelling.Health Technol Assess. 2024 Oct;28(63):1-329. doi: 10.3310/AYWA5297. Health Technol Assess. 2024. PMID: 39365169 Free PMC article.
References
-
- Lombard L, Ye W, Nichols R, Jackson J, Cotton S, Joshi S. A real-world analysis of patient characteristics, treatment patterns, and level of impairment in patients with migraine who are insufficient responders vs responders to acute treatment. Headache. 2020;60(7):1325-1339. doi:10.1111/head.13835 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

