Incidence of Complications from Percutaneous Biopsy in Chronic Liver Disease: A Systematic Review and Meta-Analysis
- PMID: 34129125
- PMCID: PMC9237012
- DOI: 10.1007/s10620-021-07089-w
Incidence of Complications from Percutaneous Biopsy in Chronic Liver Disease: A Systematic Review and Meta-Analysis
Abstract
Background: Approaches to liver biopsy have changed over the past decade in patients with chronic liver disease.
Aims: We conducted a systematic review and meta-analysis on the incidence of all complications and technical failure associated with percutaneous liver biopsy.
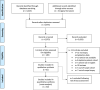
Methods: We systematically searched PubMed and the Cochrane Library for cohort studies reporting on complications resulting from liver biopsy published between 2010 and 2020. Studies on participants of any age and sex, who underwent any percutaneous biopsy for non-focal liver disease, were selected. All events except mild pain, minor hematoma, vasovagal episodes, fever and fistula were defined as major complications. Random-effect model meta-analyses with and without covariates were performed, to examine the effect of publication year, patient characteristics, outcome collection, and biopsy type on incidences.
Results: We identified 30 studies reporting on complications resulting from percutaneous liver biopsy procedures (n = 64,356). Incidence of major complications was 2.44% (95% CI 0.85, 6.75), with mortality at 0.01% (95% CI 0.00, 0.11), hospitalization at 0.65% (95% CI 0.38, 1.11), major bleeding at 0.48% (95% CI 0.22, 1.06), and moderate/severe pain at 0.34% (95% CI 0.08, 1.37). Minor complications at 9.53% (95% CI 3.68, 22.5) were mainly pain at 12.9% (95% CI 5.34, 27.9). Technical failure was high at 0.91% (95% CI 0.27, 3.00). Decreasing patient age significantly increased incidence of hospitalization and major bleeding (P < 0.0001). Hospitalization incidence also significantly increased with disease severity.
Conclusions: Incidence of major (2.4%) and minor (9.5%) complications, and technical failure (0.91%) in percutaneous liver biopsies continues.
Keywords: Bleeding; Hospitalization; Pain; Percutaneous liver biopsy.
© 2021. The Author(s).
Conflict of interest statement
No funding was received for this work. HTB, MF, CP and SF are employees of Perspectum Ltd, a privately funded commercial enterprise that develops medical devices to address unmet clinical needs, including LiverMultiScan®. DRHC is an employee of the Global Liver Institute, a non-profit organization. MN has been on the advisory board for Gilead, Intercept, Pfizer, Fractyl, Novartis, Allergan, Blade, Echosens, EchoSens North America, OWL, Siemens, Roche diagnostic and Abbott; Speaker: Abbott, Echosens, Intercept. MN has received research support from Allergan, BMS, Gilead, Galmed, Galectin, Genfit, Conatus, Enanta, Novartis, Shire and Zydus; MN is a minor shareholder or has stocks in Anaetos and Viking. All other authors have nothing to disclose.
Figures










References
-
- Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49(3):1017–1044. - PubMed
-
- Neuberger J, Patel J, Caldwell H, Davies S, Hebditch V, Hollywood C, et al. Guidelines on the use of liver Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut. 2020;69(8):1382 LP–1403. - PMC - PubMed
-
- Dezsőfi A, Baumann U, Dhawan A, Durmaz O, Fischler B, Hadzic N, et al. Liver biopsy in children: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2015;60(3):408–420. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

