Enhanced matrix production by cocultivated human stem cells and chondrocytes under concurrent mechanical strain
- PMID: 34129185
- PMCID: PMC8380185
- DOI: 10.1007/s11626-021-00592-4
Enhanced matrix production by cocultivated human stem cells and chondrocytes under concurrent mechanical strain
Abstract
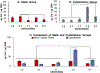
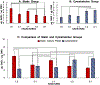
Conventional treatments of osteoarthritis have failed to re-build functional articular cartilage. Tissue engineering clinical treatments for osteoarthritis, including autologous chondrocyte implantation, provides an alternative approach by injecting a cell suspension to fill lesions within the cartilage in osteoarthritic knees. The success of chondrocyte implantation relies on the availability of chondrogenic cell lines, and their resilience to high mechanical loading. We hypothesize we can reduce the numbers of human articular chondrocytes necessary for a treatment by supplementing cultures with human adipose-derived stem cells, in which stem cells will have protective and stimulatory effects on mixed cultures when exposed to high mechanical loads, and in which coculture will enhance production of requisite extracellular matrix proteins over those produced by stretched chondrocytes alone. In this work, adipose-derived stem cells and articular chondrocytes were cultured separately or cocultivated at ratios of 3:1, 1:1, and 1:3 in static plates or under excessive cyclic tensile strain of 10% and results were compared to culturing of both cell types alone with and without cyclic strain. Results indicate 75% of chondrocytes in engineered articular cartilage can be replaced with stem cells with enhanced collagen over all culture conditions and glycosaminoglycan content over stretched cultures of chondrocytes. This can be done without observing adverse effects on cell viability. Collagen and glycosaminoglycan secretion, when compared to chondrocyte alone under 10% strain, was enhanced 6.1- and 2-fold, respectively, by chondrocytes cocultivated with stem cells at a ratio of 1:3.
Keywords: Articular cartilage; Chondrocytes; Coculture techniques; Cyclic tensile strain; Mesenchymal stem cells; Tissue engineering.
© 2021. The Society for In Vitro Biology.
Figures

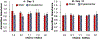
References
-
- Akagi M, Nishimura S, Yoshida K, Kakinuma T, Sawamura T, Munakata H, & Hamanishi C (2006). Cyclic tensile stretch load and oxidized low density lipoprotein synergistically induce lectin-like oxidized ldl receptor-1 in cultured bovine chondrocytes, resulting in decreased cell viability and proteoglycan synthesis. J Orthop Res, 24(8), 1782–1790. 10.1002/jor.20211 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

