Interaction between CO2-consuming autotrophy and CO2-producing heterotrophy in non-axenic phototrophic biofilms
- PMID: 34129611
- PMCID: PMC8205120
- DOI: 10.1371/journal.pone.0253224
Interaction between CO2-consuming autotrophy and CO2-producing heterotrophy in non-axenic phototrophic biofilms
Abstract
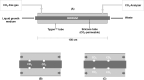
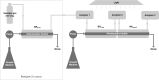
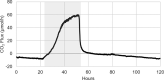
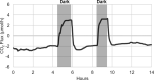
As the effects of climate change become increasingly evident, the need for effective CO2 management is clear. Microalgae are well-suited for CO2 sequestration, given their ability to rapidly uptake and fix CO2. They also readily assimilate inorganic nutrients and produce a biomass with inherent commercial value, leading to a paradigm in which CO2-sequestration, enhanced wastewater treatment, and biomass generation could be effectively combined. Natural non-axenic phototrophic cultures comprising both autotrophic and heterotrophic fractions are particularly attractive in this endeavour, given their increased robustness and innate O2-CO2 exchange. In this study, the interplay between CO2-consuming autotrophy and CO2-producing heterotrophy in a non-axenic phototrophic biofilm was examined. When the biofilm was cultivated under autotrophic conditions (i.e. no organic carbon), it grew autotrophically and exhibited CO2 uptake. After amending its growth medium with organic carbon (0.25 g/L glucose and 0.28 g/L sodium acetate), the biofilm rapidly toggled from net-autotrophic to net-heterotrophic growth, reaching a CO2 production rate of 60 μmol/h after 31 hours. When the organic carbon sources were provided at a lower concentration (0.125 g/L glucose and 0.14 g/L sodium acetate), the biofilm exhibited distinct, longitudinally discrete regions of heterotrophic and autotrophic metabolism in the proximal and distal halves of the biofilm respectively, within 4 hours of carbon amendment. Interestingly, this upstream and downstream partitioning of heterotrophic and autotrophic metabolism appeared to be reversible, as the position of these regions began to flip once the direction of medium flow (and hence nutrient availability) was reversed. The insight generated here can inform new and important research questions and contribute to efforts aimed at scaling and industrializing algal growth systems, where the ability to understand, predict, and optimize biofilm growth and activity is critical.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
The Contribution Ratio of Autotrophic and Heterotrophic Metabolism during a Mixotrophic Culture of Chlorella sorokiniana.Int J Environ Res Public Health. 2021 Feb 2;18(3):1353. doi: 10.3390/ijerph18031353. Int J Environ Res Public Health. 2021. PMID: 33540891 Free PMC article.
-
Optimizing culture conditions for heterotrophic-assisted photoautotrophic biofilm growth of Chlorella vulgaris to simultaneously improve microalgae biomass and lipid productivity.Bioresour Technol. 2018 Dec;270:80-87. doi: 10.1016/j.biortech.2018.08.116. Epub 2018 Aug 30. Bioresour Technol. 2018. PMID: 30212777
-
Legacy Effects of Plant Community Structure Are Manifested in Microbial Biofilm Development With Consequences for Ecosystem CO2 Emissions.Glob Chang Biol. 2024 Dec;30(12):e17603. doi: 10.1111/gcb.17603. Glob Chang Biol. 2024. PMID: 39611239 Free PMC article.
-
Fractional contributions by autotrophic and heterotrophic respiration to soil-surface CO2 efflux in Boreal forests.SEB Exp Biol Ser. 2005:251-67. SEB Exp Biol Ser. 2005. PMID: 17633039 Review.
-
Lipid Accumulation Mechanisms in Auto- and Heterotrophic Microalgae.J Agric Food Chem. 2017 Sep 20;65(37):8099-8110. doi: 10.1021/acs.jafc.7b03495. Epub 2017 Sep 11. J Agric Food Chem. 2017. PMID: 28838232 Review.
References
-
- Centre for Disease Control. Global WASH Fast Facts. 2016 Apr 11. In: Global Water, Sanitation, & Hygiene (WASH). Available from: https://www.cdc.gov/healthywater/global/wash_statistics.html
-
- Sutherland DL, Craggs RJ. Utilising periphytic algae as nutrient removal systems for the treatment of diffuse nutrient pollution in waterways. Algal Res. 2017;25:496–506.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

