How to deal with the consent of adults with cognitive impairment involved in European geriatric living labs?
- PMID: 34130730
- PMCID: PMC8207703
- DOI: 10.1186/s13010-021-00101-1
How to deal with the consent of adults with cognitive impairment involved in European geriatric living labs?
Abstract
Background: Living labs are realistic environments designed to create links between technology developers and end-users (i.e. mostly older adults). Research in LLH (Living labs in health) covers a wide range of studies from non-interventional studies to CT (clinical trials) and should involve patients with neurocognitive disorders. However, the ethical issues raised by the design, development, and implementation of research and development projects in LLH have been the subject of only little interest thus far.
Objective: Our aim was to determine a pragmatic, ethical and regulatory correct approach to seek the informed consent of patients with neurocognitive disorders according to the different types of studies carried out in European LLH, with a focus on the French context.
Methods: A narrative review of regulatory texts and clinical articles was conducted, and a pragmatic procedure to determine the decision-making capacity of older adults in LLH was proposed.
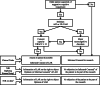
Results: Individuals must be adequately informed and freely agree to participate in CT. The capacity to consent should be assessed in CT including cognitively impaired older adults. We propose the following steps: first to assess for delirium using the 4 'A's Test (4AT) or the 3-min Diagnostic interview for Confusion Assessment Method (3D-CAM), second to search for medical history of major neurocognitive disorder, and third to assess the decision capacity using the University of California, San Diego Brief Assessment of Capacity to Consent (UBACC).
Conclusions: Including individuals with neurocognitive disorders in research implies using an efficient and pragmatic strategy to inform participants and obtain their consent. The tool we offer here may be useful in the routine operation of LLH but can also be extended to all CT with this population.
Keywords: Consent; Ethics; Geriatrics; Living lab; Older adults.
Conflict of interest statement
All authors state that they have no conflicts of interest with this paper. The authors have no relevant personal financial interest in this manuscript.
Figures
References
-
- Noublanche F, Jaglin-Grimonprez C, Laignel L, Sacco G, Allain P, Annweiler C. Adapting Gerontechnological development to hospitalized frail older people: implementation of the ALLEGRO hospital-based geriatric living lab. J Am Med Dir Assoc. 2020;21(4):550–554. doi: 10.1016/j.jamda.2020.01.001. - DOI - PubMed
-
- Kim J, Kim YL, Jang H, Cho M, Lee M, Kim J, et al. Living labs for health: an integrative literature review. Eur J Pub Health. 2020;30(1):55–63. 10.1093/eurpub/ckz105. - PubMed
-
- Habibipour A, Stahlbrost A, Georges A, Bergvall-Kåreborn B. Drop-out in living lab field test : analyzing consequences and some recommendations. Twenty-Sixth European Conference on Information Systems (ECIS2018), Portsmouth, UK, 2018. Presented at the 26th European Conference on Information Systems (ECIS2018), Portsmouth, UK, 23–28 June 2018. Retrieved from http://urn.kb.se/resolve?urn=urn:nbn:se:ltu:diva-69367.
-
- Sainz F. Emerging ethical issues in living labs. Ramon Llull J Appl Ethics. 2012;3(3):47. doi: 10.4103/2013-8393.107298. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

