Donor-derived cell-free DNA accurately detects acute rejection in lung transplant patients, a multicenter cohort study
- PMID: 34130911
- PMCID: PMC8319066
- DOI: 10.1016/j.healun.2021.04.009
Donor-derived cell-free DNA accurately detects acute rejection in lung transplant patients, a multicenter cohort study
Abstract
Background: Acute rejection, which includes antibody-mediated rejection and acute cellular rejection, is a risk factor for lung allograft loss. Lung transplant patients often undergo surveillance transbronchial biopsies to detect and treat acute rejection before irreversible chronic rejection develops. Limitations of this approach include its invasiveness and high interobserver variability. We tested the performance of percent donor-derived cell-free DNA (%ddcfDNA), a non-invasive blood test, to detect acute rejection.
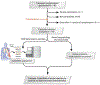
Methods: This multicenter cohort study monitored 148 lung transplant subjects over a median of 19.6 months. We collected serial plasma samples contemporaneously with TBBx to measure %ddcfDNA. Clinical data was collected to adjudicate for acute rejection. The primary analysis consisted of computing the area-under-the-receiver-operating-characteristic-curve of %ddcfDNA to detect acute rejection. Secondary analysis determined %ddcfDNA rule-out thresholds for acute rejection.
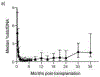
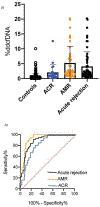
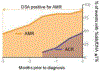
Results: ddcfDNA levels were high after transplant surgery and decayed logarithmically. With acute rejection, ddcfDNA levels rose six-fold higher than controls. ddcfDNA levels also correlated with severity of lung function decline and histological grading of rejection. %ddcfDNA area-under-the-receiver-operating-characteristic-curve for acute rejection, AMR, and ACR were 0.89, 0.93, and 0.83, respectively. ddcfDNA levels of <0.5% and <1.0% showed a negative predictive value of 96% and 90% for acute rejection, respectively. Histopathology detected one-third of episodes with ddcfDNA levels ≥1.0%, even though >90% of these events were coincident to clinical complications missed by histopathology.
Conclusions: This study demonstrates that %ddcfDNA reliably detects acute rejection and other clinical complications potentially missed by histopathology, lending support to its use as a non-invasive marker of allograft injury.
Keywords: cell-free DNA; early diagnosis; rejection.
Copyright © 2021 International Society for Heart and Lung Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no competing interests to disclose.
Figures
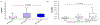
References
-
- Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26(12):1229–42. - PubMed
-
- Roux A, Levine DJ, Zeevi A, Hachem R, Halloran K, Halloran PF, et al. Banff Lung Report: Current knowledge and future research perspectives for diagnosis and treatment of pulmonary antibody-mediated rejection (AMR). Am J Transplant. 2019;19(1):21–31. - PubMed
-
- Roux A, Bendib Le Lan I, Holifanjaniaina S, Thomas KA, Hamid AM, Picard C, et al. Antibody-Mediated Rejection in Lung Transplantation: Clinical Outcomes and Donor-Specific Antibody Characteristics. Am J Transplant. 2016;16(4):1216–28. - PubMed
-
- Sato M, Ohmori-Matsuda K, Saito T, Matsuda Y, Hwang DM, Waddell TK, et al. Time-dependent changes in the risk of death in pure bronchiolitis obliterans syndrome (BOS). J Heart Lung Transplant. 2013;32(5):484–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

