Baseline Predictors of Glycemic Worsening in Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes in the Restoring Insulin Secretion (RISE) Study
- PMID: 34131048
- PMCID: PMC8740917
- DOI: 10.2337/dc21-0027
Baseline Predictors of Glycemic Worsening in Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes in the Restoring Insulin Secretion (RISE) Study
Abstract
Objective: To identify predictors of glycemic worsening among youth and adults with impaired glucose tolerance (IGT) or recently diagnosed type 2 diabetes in the Restoring Insulin Secretion (RISE) Study.
Research design and methods: A total of 91 youth (10-19 years) were randomized 1:1 to 12 months of metformin (MET) or 3 months of glargine, followed by 9 months of metformin (G-MET), and 267 adults were randomized to MET, G-MET, liraglutide plus MET (LIRA+MET), or placebo for 12 months. All participants underwent a baseline hyperglycemic clamp and a 3-h oral glucose tolerance test (OGTT) at baseline, month 6, month 12, and off treatment at month 15 and month 21. Cox models identified baseline predictors of glycemic worsening (HbA1c increase ≥0.5% from baseline).
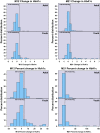
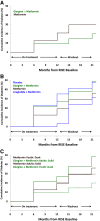
Results: Glycemic worsening occurred in 17.8% of youth versus 7.5% of adults at month 12 (P = 0.008) and in 36% of youth versus 20% of adults at month 21 (P = 0.002). In youth, glycemic worsening did not differ by treatment. In adults, month 12 glycemic worsening was less on LIRA+MET versus placebo (hazard ratio 0.21, 95% CI 0.05-0.96, P = 0.044). In both age-groups, lower baseline clamp-derived β-cell responses predicted month 12 and month 21 glycemic worsening (P < 0.01). Lower baseline OGTT-derived β-cell responses predicted month 21 worsening (P < 0.05). In youth, higher baseline HbA1c and 2-h glucose predicted month 12 and month 21 glycemic worsening, and higher fasting glucose predicted month 21 worsening (P < 0.05). In adults, lower clamp- and OGTT-derived insulin sensitivity predicted month 12 and month 21 worsening (P < 0.05).
Conclusions: Glycemic worsening was more common among youth than adults with IGT or recently diagnosed type 2 diabetes, predicted by lower baseline β-cell responses in both groups, hyperglycemia in youth, and insulin resistance in adults.
Trial registration: ClinicalTrials.gov NCT01779362 NCT01779375.
© 2021 by the American Diabetes Association.
Figures
References
-
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 - PubMed
-
- Love-Osborne KA, Sheeder JL, Nadeau KJ, Zeitler P. Longitudinal follow up of dysglycemia in overweight and obese pediatric patients. Pediatr Diabetes 2018;19:199–204 - PubMed
-
- Turner RC, Cull CA, Frighi V; UK Prospective Diabetes Study (UKPDS) Group . Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–2012 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 TR000430/TR/NCATS NIH HHS/United States
- U01 DK094406/DK/NIDDK NIH HHS/United States
- P30 DK048520/DK/NIDDK NIH HHS/United States
- UL1 TR001857/TR/NCATS NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U01 DK094431/DK/NIDDK NIH HHS/United States
- U01 DK094430/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- P30 DK020595/DK/NIDDK NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- TL1 TR001858/TR/NCATS NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- U01 DK094438/DK/NIDDK NIH HHS/United States
- P30 DK116073/DK/NIDDK NIH HHS/United States
- U01 DK094467/DK/NIDDK NIH HHS/United States
- P30 DK097512/DK/NIDDK NIH HHS/United States
- K24 HL145076/HL/NHLBI NIH HHS/United States
- UL1 TR001855/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

