Differential Binding of Autoantibodies to MOG Isoforms in Inflammatory Demyelinating Diseases
- PMID: 34131067
- PMCID: PMC8207634
- DOI: 10.1212/NXI.0000000000001027
Differential Binding of Autoantibodies to MOG Isoforms in Inflammatory Demyelinating Diseases
Abstract
Objective: To analyze serum immunoglobulin G (IgG) antibodies to major isoforms of myelin oligodendrocyte glycoprotein (MOG-alpha 1-3 and beta 1-3) in patients with inflammatory demyelinating diseases.
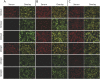
Methods: Retrospective case-control study using 378 serum samples from patients with multiple sclerosis (MS), patients with non-MS demyelinating disease, and healthy controls with MOG alpha-1-IgG positive (n = 202) or negative serostatus (n = 176). Samples were analyzed for their reactivity to human, mouse, and rat MOG isoforms with and without mutations in the extracellular MOG Ig domain (MOG-ecIgD), soluble MOG-ecIgD, and myelin from multiple species using live cell-based, tissue immunofluorescence assays and ELISA.
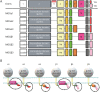
Results: The strongest IgG reactivities were directed against the longest MOG isoforms alpha-1 (the currently used standard test for MOG-IgG) and beta-1, whereas the other isoforms were less frequently recognized. Using principal component analysis, we identified 3 different binding patterns associated with non-MS disease: (1) isolated reactivity to MOG-alpha-1/beta-1 (n = 73), (2) binding to MOG-alpha-1/beta-1 and at least one other alpha, but no beta isoform (n = 64), and (3) reactivity to all 6 MOG isoforms (n = 65). The remaining samples were negative (n = 176) for MOG-IgG. These MOG isoform binding patterns were associated with a non-MS demyelinating disease, but there were no differences in clinical phenotypes or disease course. The 3 MOG isoform patterns had distinct immunologic characteristics such as differential binding to soluble MOG-ecIgD, sensitivity to MOG mutations, and binding to human MOG in ELISA.
Conclusions: The novel finding of differential MOG isoform binding patterns could inform future studies on the refinement of MOG-IgG assays and the pathophysiologic role of MOG-IgG.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures



References
-
- Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. 2019;15(2):89-102. - PubMed
-
- Cobo-Calvo A, Ruiz A, Maillart E, et al. . Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology. 2018;90(21):e1858-e1869. - PubMed
-
- Jurynczyk M, Messina S, Woodhall MR, et al. . Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140(12):3128-3138. - PubMed
-
- Hennes EM, Baumann M, Schanda K, et al. . Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology. 2017;89(9):900-908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
