Comparative Cost Effectiveness of Reflux-Based and Reflux-Independent Strategies for Barrett's Esophagus Screening
- PMID: 34131096
- PMCID: PMC8315187
- DOI: 10.14309/ajg.0000000000001336
Comparative Cost Effectiveness of Reflux-Based and Reflux-Independent Strategies for Barrett's Esophagus Screening
Abstract
Introduction: Minimally invasive tests for Barrett's esophagus (BE) detection have raised the prospect of broader nonreflux-based testing. Cost-effectiveness studies have largely studied men aged 50 years with chronic gastroesophageal reflux disease (GERD) symptoms. We evaluated the comparative cost effectiveness of BE screening tests in GERD-based and GERD-independent testing scenarios.
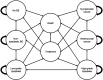
Methods: Markov modeling was performed in 3 scenarios in 50 years old individuals: (i) White men with chronic GERD (GERD-based); (ii) GERD-independent (all races, men and women), BE prevalence 1.6%; and (iii) GERD-independent, BE prevalence 5%. The simulation compared multiple screening strategies with no screening: sedated endoscopy (sEGD), transnasal endoscopy, swallowable esophageal cell collection devices with biomarkers, and exhaled volatile organic compounds. A hypothetical cohort of 500,000 individuals followed for 40 years using a willingness to pay threshold of $100,000 per quality-adjusted life year (QALY) was simulated. Incremental cost-effectiveness ratios (ICERs) comparing each strategy with no screening and comparing screening strategies with each other were calculated.
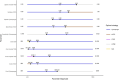
Results: In both GERD-independent scenarios, most non-sEGD BE screening tests were cost effective. Swallowable esophageal cell collection devices with biomarkers were cost effective (<$35,000/QALY) and were the optimal screening tests in all scenarios. Exhaled volatile organic compounds had the highest ICERs in all scenarios. ICERs were low (<$25,000/QALY) for all tests in the GERD-based scenario, and all non-sEGD tests dominated no screening. ICERs were sensitive to BE prevalence and test costs.
Discussion: Minimally invasive nonendoscopic tests may make GERD-independent BE screening cost effective. Participation rates for these strategies need to be studied.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures



References
-
- Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: Are we reaching the peak?. Cancer Epidemiol Biomarkers Prev 2010;19:1468–70. - PubMed
-
- Garside R, Pitt M, Somerville M, et al. Surveillance of Barrett's oesophagus: Exploring the uncertainty through systematic review, expert workshop and economic modelling. Health Technology Assess 2006;10:iii–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

