Immunogenicity and safety of a tetravalent dengue vaccine in dengue-naïve adolescents in Mexico City
- PMID: 34131423
- PMCID: PMC8196333
- DOI: 10.26633/RPSP.2021.67
Immunogenicity and safety of a tetravalent dengue vaccine in dengue-naïve adolescents in Mexico City
Abstract
Objective: To describe the immunogenicity and safety of a tetravalent dengue vaccine (TAK-003) in healthy adolescents living in Mexico City, an area considered non-endemic for dengue (NCT03341637).
Methods: Participants aged 12-17 years were randomized 3:1 to receive two doses (Month 0 and Month 3) of TAK-003 or placebo. Immunogenicity was assessed by microneutralization assay of dengue neutralizing antibodies at baseline, Months 4 and 9. Solicited and unsolicited adverse events (AEs) were recorded after each vaccination. Serious (SAEs) and medically-attended AEs (MAAEs) were recorded throughout the study.
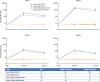
Results: 400 adolescents were enrolled, 391 (97.8%) completed the study. Thirty-six (9%) were baseline seropositive to ≥1 serotypes (reciprocal titer ≥10). Geometric mean titers (GMTs) in baseline seronegative TAK-003 recipients were 328, 1743, 120, and 143 at Month 4, and 135, 741, 46, and 38 at Month 9 against DENV-1, -2, -3, and -4, respectively. Placebo GMTs remained <10. Tetravalent seropositivity rates in vaccine recipients were 99.6% and 85.8% at Months 4 and 9, respectively. One MAAE in each group was considered treatment-related (TAK-003: injection-site erythema, and placebo: pharyngitis).
Conclusion: TAK-003 was immunogenic against all four serotypes and was well tolerated in dengue-naïve adolescents living in Mexico City.
Objetivo: Describir la inmunogenicidad y la seguridad de una vacuna tetravalente contra el dengue (TAK-003) en adolescentes sanos residentes en Ciudad de México, considerada un área no endémica de dengue (NCT03341637).
Métodos: Se asignó de manera aleatoria a un grupo de participantes de 12 a 17 años en una proporción 3:1 para que recibieran dos dosis (en el mes 0 y en el mes 3) de la vacuna TAK-003 o de un placebo. Se evaluó la inmunogenicidad mediante un análisis de microneutralización de anticuerpos neutralizantes del virus del dengue al inicio del estudio y en los meses 4 y 9. Se registraron los eventos adversos de notificación solicitada y los referidos por iniciativa propia después de cada vacunación. A lo largo del estudio se registraron los eventos adversos graves y los que requirieron atención médica.
Resultados: Participaron 400 adolescentes y 391 (97,8%) finalizaron el estudio. 36 adolescentes (9%) fueron seropositivos a ≥1 serotipos (título recíproco ≥10) al inicio del estudio. La media geométrica de los títulos en las personas seronegativas vacunadas con TAK-003 al inicio del estudio fue de 328, 1743, 120 y 143 en el mes 4 y 135, 741, 46 y 38 en el mes 9 en relación con DENV-1, -2, -3 y -4, respectivamente. La media geométrica de los títulos de las personas que recibieron un placebo se mantuvo en <10. Las tasas de seropositividad tetravalente en los vacunados fueron 99,6% y 85,8% a los meses 4 y 9, respectivamente. Se consideró relacionado con el tratamiento un evento adverso con atención médica que tuvo lugar en cada grupo (TAK-003: eritema en el lugar de la inyección; placebo: faringitis).
Conclusiones: TAK-003 fue inmunogénica ante los cuatro serotipos y bien tolerada en los adolescentes sin exposición previa al dengue que vivían en Ciudad de México.
Objetivo: Descrever a imunogenicidade e a segurança de uma vacina tetravalente contra dengue (TAK-003) em adolescentes saudáveis residentes da Cidade do México, área considerada não endêmica para dengue (ClinicalTrials.gov: NCT03341637).
Métodos: Participantes com idade entre 12 e 17 anos foram randomizados a uma proporção de 3:1 para receber duas doses da vacina TAK-003 ou placebo (no mês 0 e no mês 3). A imunogenicidade foi avaliada pelos títulos de anticorpos neutralizantes contra dengue determinados em ensaio de microneutralização ao início do estudo, no mês 4 e no mês 9. A ocorrência de eventos adversos solicitados ou espontâneos foi registrada após cada rodada de vacinação. Eventos adversos graves e eventos adversos que exigiram atendimento médico foram monitorados ao longo de todo o estudo.
Resultados: De 400 adolescentes incluídos na amostra estudada, 391 (97,8%) completaram o estudo. Trinta e seis (9%) apresentaram positividade basal a um ou mais sorotipos virais da dengue (título recíproco ≥10). A média geométrica dos títulos de anticorpos nos vacinados com TAK-003 que eram soronegativos ao início do estudo foi de 328, 1743, 120 e 143 no mês 4 e 135, 741, 46 e 38 no mês 9, contra os sorotipos virais DENV-1, DENV-2, DENV-3 e DENV-4, respectivamente. A média geométrica dos títulos de anticorpos no grupo placebo se manteve abaixo de 10. A taxa de soropositividade tetravalente nos vacinados foi de 99,6% no mês 4 e 85,8% no mês 9. Um único evento adverso que exigiu atendimento médico em cada grupo foi considerado relacionado ao tratamento (eritema no local de aplicação no grupo TAK-003 e faringite no grupo placebo).
Conclusão: A vacina TAK-003 demonstrou ser imunogênica contra os quatro sorotipos virais da dengue e foi bem tolerada em adolescentes residentes da Cidade do México sem história pregressa de infecção pela dengue.
Keywords: Mexico; Vaccines; adolescents; dengue; immunogenicity; safety.
Figures

Similar articles
-
Immunogenicity, Safety and Efficacy of the Dengue Vaccine TAK-003: A Meta-Analysis.Vaccines (Basel). 2024 Jul 13;12(7):770. doi: 10.3390/vaccines12070770. Vaccines (Basel). 2024. PMID: 39066408 Free PMC article. Review.
-
A Phase 1, double-blind, randomized, placebo-controlled study to evaluate the safety and immunogenicity of a tetravalent live attenuated dengue vaccine in adults.Vaccine. 2023 Aug 31;41(38):5614-5621. doi: 10.1016/j.vaccine.2023.07.045. Epub 2023 Jul 31. Vaccine. 2023. PMID: 37532611 Clinical Trial.
-
Safety and immunogenicity of one versus two doses of Takeda's tetravalent dengue vaccine in children in Asia and Latin America: interim results from a phase 2, randomised, placebo-controlled study.Lancet Infect Dis. 2017 Jun;17(6):615-625. doi: 10.1016/S1473-3099(17)30166-4. Epub 2017 Mar 30. Lancet Infect Dis. 2017. PMID: 28365225 Clinical Trial.
-
Immunogenicity and safety of one versus two doses of tetravalent dengue vaccine in healthy children aged 2-17 years in Asia and Latin America: 18-month interim data from a phase 2, randomised, placebo-controlled study.Lancet Infect Dis. 2018 Feb;18(2):162-170. doi: 10.1016/S1473-3099(17)30632-1. Epub 2017 Nov 6. Lancet Infect Dis. 2018. PMID: 29122463 Clinical Trial.
-
Development of a recombinant, chimeric tetravalent dengue vaccine candidate.Vaccine. 2015 Dec 10;33(50):7112-20. doi: 10.1016/j.vaccine.2015.11.022. Epub 2015 Nov 14. Vaccine. 2015. PMID: 26585500 Review.
Cited by
-
Immunogenicity, Safety and Efficacy of the Dengue Vaccine TAK-003: A Meta-Analysis.Vaccines (Basel). 2024 Jul 13;12(7):770. doi: 10.3390/vaccines12070770. Vaccines (Basel). 2024. PMID: 39066408 Free PMC article. Review.
-
Clinical Safety Experience of TAK-003 for Dengue Fever: A New Tetravalent Live Attenuated Vaccine Candidate.Clin Infect Dis. 2023 Feb 8;76(3):e1350-e1359. doi: 10.1093/cid/ciac418. Clin Infect Dis. 2023. PMID: 35639602 Free PMC article. Clinical Trial.
-
Efficacy and Safety of a Tetravalent Dengue Vaccine (TAK-003) in Children With Prior Japanese Encephalitis or Yellow Fever Vaccination.J Infect Dis. 2024 Dec 16;230(6):e1214-e1225. doi: 10.1093/infdis/jiae222. J Infect Dis. 2024. PMID: 38682569 Free PMC article. Clinical Trial.
-
Exploring plant-based dengue therapeutics: from laboratory to clinic.Trop Dis Travel Med Vaccines. 2024 Nov 15;10(1):23. doi: 10.1186/s40794-024-00232-1. Trop Dis Travel Med Vaccines. 2024. PMID: 39543749 Free PMC article.
-
A Maximum Entropy Model of the Distribution of Dengue Serotype in Mexico.Transbound Emerg Dis. 2023 Jul 25;2023:3823879. doi: 10.1155/2023/3823879. eCollection 2023. Transbound Emerg Dis. 2023. PMID: 40303721 Free PMC article.
References
-
- World Health Organization . Geneva: WHO; 2020. [Accessed 05 June 2020]. Urgent health challenges for the next decade [Internet] Available from: https://www.who.int/news-room/photo-story/photo-story-detail/urgent-heal....
- 1. World Health Organization. Urgent health challenges for the next decade [Internet]. Geneva: WHO; 2020. Available from: https://www.who.int/news-room/photo-story/photo-story-detail/urgent-heal.... Accessed 05 June 2020.
-
- World Health Organization . Geneva: WHO; 2019. [Accessed 04 November 2019]. Dengue and Severe Dengue Fact Sheet [Internet] Avaliable from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- 2. World Health Organization. Dengue and Severe Dengue Fact Sheet [Internet]. Geneva: WHO; 2019. Avaliable from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. Accessed 04 November 2019.
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. - DOI - PMC - PubMed
- 3. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504-7. doi: 10.1038/nature12060. - PMC - PubMed
-
- Wilder-Smith A. Dengue infections in travellers. Paediatr Int Child Health. 2012;32(Suppl 1):28–32. doi: 10.1179/2046904712Z.00000000050. - DOI - PMC - PubMed
- 4. Wilder-Smith A. Dengue infections in travellers. Paediatr Int Child Health. 2012;32 Suppl 1:28-32. doi: 10.1179/2046904712Z.00000000050. - PMC - PubMed
-
- Halstead S, Wilder-Smith A. Severe dengue in travellers: pathogenesis, risk and clinical management. J Travel Med. 2019;26(7):taz062. doi: 10.1093/jtm/taz062. - DOI - PubMed
- 5. Halstead S, Wilder-Smith A. Severe dengue in travellers: pathogenesis, risk and clinical management. J Travel Med. 2019;26(7):taz062. doi: 10.1093/jtm/taz062. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
