Dracorhodin perchlorate enhances wound healing via β-catenin, ERK/p38, and AKT signaling in human HaCaT keratinocytes
- PMID: 34131445
- PMCID: PMC8193218
- DOI: 10.3892/etm.2021.10254
Dracorhodin perchlorate enhances wound healing via β-catenin, ERK/p38, and AKT signaling in human HaCaT keratinocytes
Abstract
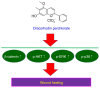
Dracorhodin can be isolated from the exudates of the fruit of Daemonorops draco. Previous studies suggested that dracorhodin perchlorate can promote fibroblast proliferation and enhance angiogenesis during wound healing. In the present study, the potential bioactivity of dracorhodin perchlorate in human HaCaT keratinocytes, were investigated in vitro, with specific focus on HaCaT wound healing. The results of in vitro scratch assay demonstrated the progressive closure of the wound after treatment with dracorhodin perchlorate in a time-dependent manner. An MTT assay and propidium iodide exclusion detected using flow cytometry were used to detect cell viability of HaCaT cells. Potential signaling pathways underlying the effects mediated by dracorhodin perchlorate in HaCaT cells were clarified by western blot analysis and kinase activity assays. Dracorhodin perchlorate significantly increased the protein expression levels of β-catenin and activation of AKT, ERK and p38 in HaCaT cells. In addition, dracorhodin perchlorate did not induce HaCaT cell proliferation but promoted cell migration. Other mechanisms may yet be involved in the dracorhodin perchlorate-induced wound healing process of human keratinocytes. In summary, dracorhodin perchlorate may serve to be a potential molecularly-targeted phytochemical that can improve skin wound healing.
Keywords: ERK/p38/AKT signaling; HaCaT keratinocytes; dracorhodin perchlorate. β-catenin; wound healing.
Copyright: © Lu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
LinkOut - more resources
Full Text Sources
Miscellaneous
