Recent advances in the understanding and management of hepatorenal syndrome
- PMID: 34131658
- PMCID: PMC8170686
- DOI: 10.12703/r/10-48
Recent advances in the understanding and management of hepatorenal syndrome
Abstract
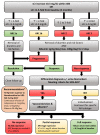
Renal dysfunction occurs frequently in hospitalized patients with advanced chronic liver disease (ACLD)/cirrhosis and has profound prognostic implications. In ACLD patients with ascites, hepatorenal syndrome (HRS) may result from circulatory dysfunction that leads to reduced kidney perfusion and glomerular filtration rate (in the absence of structural kidney damage). The traditional subclassification of HRS has recently been replaced by acute kidney injury (AKI) type of HRS (HRS-AKI) and non-AKI type of HRS (HRS-NAKI), replacing the terms "HRS type 1" and "HRS type 2", respectively. Importantly, the concept of absolute serum creatinine (sCr) cutoffs for diagnosing HRS was partly abandoned and short term sCr dynamics now may suffice for AKI diagnosis, which facilitates early treatment initiation that may prevent the progression to HRS-AKI or increase the chances of AKI/HRS-AKI reversal. Recent randomized controlled trials have established (a) the efficacy of (long-term) albumin in the prevention of complications of ascites (including HRS-AKI), (b) the benefits of transjugular intrahepatic portosystemic shunt placement in patients with recurrent ascites, and (c) the superiority of terlipressin over noradrenaline for the treatment of HRS-AKI in the context of acute-on-chronic liver failure. This review article aims to summarize recent advances in the understanding and management of HRS.
Keywords: acute kidney injury; ascites; cirrhosis; portal hypertension; renal impairment.
Copyright: © 2021 Mandorfer M et al.
Conflict of interest statement
BS received travel support from AbbVie and Gilead. MT received grant support from Albireo, Cymabay, Falk, Gilead, Intercept, MSD, and Takeda; consulting honoraria from BiomX, Boehringer Ingelheim, Falk, Genfit, Gilead, Intercept, Janssen, MSD, Novartis, Phenex, and Regulus; speaker fees from BMS, Falk, Gilead, Intercept, and MSD; and travel support from AbbVie, Falk, Gilead, and Intercept. TR received grant support from AbbVie, Boehringer Ingelheim, Gilead, MSD, Philips Healthcare, and Gore; speaking honoraria from AbbVie, Gilead, Gore, Intercept, Roche, and MSD; consulting or advisory board fees from AbbVie, Bayer, Boehringer Ingelheim, Gilead, Intercept, MSD, and Siemens; and travel support from AbbVie, Boehringer Ingelheim, Gilead, and Roche. MM served as a speaker, consultant, or advisory board member (or a combination of these) for AbbVie, Bristol Myers Squibb, Collective Acumen, Gilead, and W. L. Gore & Associates and received travel support from AbbVie, Bristol Myers Squibb, and Gilead.No competing interests were disclosed.No competing interests were disclosed.
Figures


References
-
- Bellomo R, Ronco C, Kellum JA, et al. : Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8(4): R204–12. 10.1186/cc2872 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
