Adenomyosis pathogenesis: insights from next-generation sequencing
- PMID: 34131719
- PMCID: PMC8543024
- DOI: 10.1093/humupd/dmab017
Adenomyosis pathogenesis: insights from next-generation sequencing
Abstract
Background: Adenomyosis, characterized by the presence of islands of endometrial tissue surrounded by hypertrophic smooth muscle cells within the myometrium, is one of the most challenging uterine disorders in terms of diagnosis and management. Adenomyosis presents with pelvic pain, excessive uterine bleeding, anemia and infertility. The relative contributions of abnormal endometrial tissue and myometrial smooth muscle cells to the development and growth of adenomyosis are not well understood. Moreover, there is continuing debate on the origins of adenomyosis; two competing theories describe the invagination of basal endometrium into the myometrium or the metaplastic differentiation of remnant endometrial stem/progenitor cells within the myometrium.
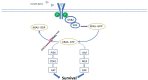
Objective and rationale: A recent series of next-generation sequencing (NGS) studies have provided the best scientific evidence thus far regarding the cellular origins of adenomyosis and the contributions of new signaling pathways to its pathogenesis, survival, and growth. These seminal studies on endometrium, adenomyosis and endometriosis demonstrate or support the following key points. (i) Mutations of KRAS map to both intracavitary endometrial tissue and proximally located adenomyotic samples, supporting the invagination theory of pathogenesis. Driver mutations found in smooth muscle cells of uterine fibroids are absent in adenomyosis. (ii) KRAS and other less frequent mutations are limited to endometrial-type epithelial cells. They are also observed in endometriosis, indicating that the disease process in adenomyosis is similar to that in endometriosis and distinct from that of uterine fibroids. (iii) Activating mutations of KRAS stimulate specific pathways to increase cell survival and proliferation and are associated with progesterone resistance in adenomyosis. Together, these findings suggest that distinct cell populations in eutopic endometrial tissue play key roles in the etiology of adenomyosis. Dependence on ovarian steroids and ovulatory cycles for disease severity is a unique feature of adenomyosis. In this context, common patterns of aberrant gene expression have been reported both in adenomyosis and endometriosis. These include pathways that favor increased estrogen biosynthesis, decreased estradiol metabolism, a unique estrogen receptor beta (ESR2)-driven inflammatory process, and progesterone resistance due to decreased progesterone receptor expression. Since adenomyosis exhibits a uniquely estrogen-driven inflammatory process and progesterone resistance, we discuss the interactions between these molecular characteristics and signaling pathways induced by the newly discovered KRAS mutations.
Search methods: We conducted a comprehensive search using PubMed for human and animal studies published until 2020 in the following areas: adenomyosis, endometriosis, endometrium, NGS, whole-exome sequencing, whole-genome sequencing, RNA sequencing, targeted deep sequencing, epigenetics, driver mutation, KRAS, progesterone resistance, estrogen action and steroid production.
Outcomes: Targeted deep sequencing analyses of epithelial cells in adenomyosis and adjacent basalis endometrial glands demonstrated recurring KRAS mutations in both cell types. This finding suggests that adenomyosis originates from basalis endometrium. Epithelial cells of the endometrium, adjacent adenomyosis and co-occurring endometriosis also share identical KRAS mutations. These findings suggest both adenomyosis and endometriosis are oligoclonal tissues that arise from endometrial cell populations carrying a specific driver mutation that most commonly affects the KRAS gene.
Wider implications: Adenomyosis usually follows an event such as pregnancy that has disrupted the integrity of the endometrial-myometrial junction followed by repetitious menstrual episodes that increase the likelihood of the entrapment of the basalis endometrium within the myometrium. Glandular epithelial cells carrying KRAS mutations and located within the deep crypts of basalis endometrium may become entrapped and invade myometrial tissue to give rise to adenomyosis. Evidence suggests that KRAS mutations may be responsible, in part, for previously observed phenomena such as prolonged cell survival and progesterone resistance in adenomyosis.
Keywords: ESR1; KRAS; NGS; PGR; adenomyosis; driver mutation; endometriosis; endometrium; next-generation sequencing; progesterone resistance.
© The Author(s) 2021. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures


References
-
- Bazot M, Cortez A, Darai E, Rouger J, Chopier J, Antoine JM, Uzan S.. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod 2001;16:2427–2433. - PubMed
-
- Benagiano G, Brosens I.. History of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006;20:449–463. - PubMed
-
- Benagiano G, Brosens I.. The endometrium in adenomyosis. Womens Health (Lond) 2012;8:301–312. - PubMed
-
- Bergeron C, Amant F, Ferenczy A.. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006;20:511–521. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

