Nanobodies: new avenue to treat kidney disease
- PMID: 34131806
- PMCID: PMC8205650
- DOI: 10.1007/s00441-021-03479-8
Nanobodies: new avenue to treat kidney disease
Abstract
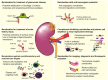
Current therapeutic options for renal diseases are limited, and the search for disease-specific treatments is ongoing. Nanobodies, single-domain antibodies with many advantages over conventional antibodies, provide flexible, easy-to-format biologicals with many possible applications. Here, we discuss the potential use of nanobodies for renal diseases.
Keywords: Conventional antibodies; Nanobodies; Renal diseases.
© 2021. The Author(s).
Conflict of interest statement
F.K.-N. is a co-inventor on patent applications on CD38-specific and P2X7-specific nanobodies.
Figures

References
-
- Balhuizen A, Massa S, Mathijs I, Turatsinze JV, De Vos J, Demine S, Xavier C, Villate O, Millard I, Egrise D, Capito C, Scharfmann R, In't Veld P, Marchetti P, Muyldermans S, Goldman S, Lahoutte T, Bouwens L, Eizirik DL, Devoogdt N. A nanobody-based tracer targeting DPP6 for non-invasive imaging of human pancreatic endocrine cells. Sci Rep. 2017;7:15130. doi: 10.1038/s41598-017-15417-2. - DOI - PMC - PubMed
-
- Bannas P, Lenz A, Kunick V, Well L, Fumey W, Rissiek B, Haag F, Schmid J, Schutze K, Eichhoff A, Trepel M, Adam G, Ittrich H, Koch-Nolte F. Molecular imaging of tumors with nanobodies and antibodies: timing and dosage are crucial factors for improved in vivo detection. Contrast Media Mol Imaging. 2015;10:367–378. doi: 10.1002/cmmi.1637. - DOI - PubMed
-
- Bannas P, Well L, Lenz A, Rissiek B, Haag F, Schmid J, Hochgrafe K, Trepel M, Adam G, Ittrich H, Koch-Nolte F. In vivo near-infrared fluorescence targeting of T cells: comparison of nanobodies and conventional monoclonal antibodies. Contrast Media Mol Imaging. 2014;9:135–142. doi: 10.1002/cmmi.1548. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

