The common ABCA3E292V variant disrupts AT2 cell quality control and increases susceptibility to lung injury and aberrant remodeling
- PMID: 34132118
- PMCID: PMC8410113
- DOI: 10.1152/ajplung.00400.2020
The common ABCA3E292V variant disrupts AT2 cell quality control and increases susceptibility to lung injury and aberrant remodeling
Abstract
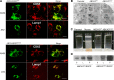
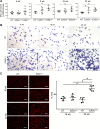
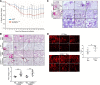
ATP-binding cassette class A3 (ABCA3) is a lipid transporter that plays a critical role in pulmonary surfactant function. The substitution of valine for glutamic acid at codon 292 (E292V) produces a hypomorphic variant that accounts for a significant portion of ABCA3 mutations associated with lung disorders spanning from neonatal respiratory distress syndrome and childhood interstitial lung disease to diffuse parenchymal lung disease (DPLD) in adults including pulmonary fibrosis. The mechanisms by which this and similar ABCA3 mutations disrupt alveolar type 2 (AT2) cell homeostasis and cause DPLD are largely unclear. The present study, informed by a patient homozygous for the E292V variant, used an in vitro and a preclinical murine model to evaluate the mechanisms by which E292V expression promotes aberrant lung injury and parenchymal remodeling. Cell lines stably expressing enhanced green fluorescent protein (EGFP)-tagged ABCA3 isoforms show a functional deficiency of the ABCA3E292V variant as a lipid transporter. AT2 cells isolated from mice constitutively homozygous for ABCA3E292V demonstrate the presence of small electron-dense lamellar bodies, time-dependent alterations in macroautophagy, and induction of apoptosis. These changes in AT2 cell homeostasis are accompanied by a spontaneous lung phenotype consisting of both age-dependent inflammation and fibrillary collagen deposition in alveolar septa. Older ABCA3E292V mice exhibit increased vulnerability to exogenous lung injury by bleomycin. Collectively, these findings support the hypothesis that the ABCA3E292V variant is a susceptibility factor for lung injury through effects on surfactant deficiency and impaired AT2 cell autophagy.
Keywords: ABCA3; autophagy; diffuse parenchymal lung disease; lung epithelium; pulmonary fibrosis.
Conflict of interest statement
M.F. Beers is an established investigator of the Pulmonary Fibrosis Foundation. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures




References
-
- Fitzgerald ML, Xavier R, Haley KJ, Welti R, Goss JL, Brown CE, Zhuang DZ, Bell SA, Lu N, McKee M, Seed B, Freeman MW. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J Lipid Res 48: 621–632, 2007. doi:10.1194/jlr.M600449-JLR200. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

