Capsid Proteins Are Necessary for Replication of a Parvovirus
- PMID: 34132571
- PMCID: PMC8354234
- DOI: 10.1128/JVI.00523-21
Capsid Proteins Are Necessary for Replication of a Parvovirus
Abstract
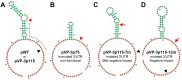
Despite tight genetic compression, viral genomes are often organized into functional gene clusters, a modular structure that might favor their evolvability. This has greatly facilitated biotechnological developments such as the recombinant adeno-associated virus (AAV) systems for gene therapy. Following this lead, we endeavored to engineer the related insect parvovirus Junonia coenia densovirus (JcDV) to create addressable vectors for insect pest biocontrol. To enable safer manipulation of capsid mutants, we translocated the nonstructural (ns) gene cluster outside the viral genome. To our dismay, this yielded a virtually nonreplicable clone. We linked the replication defect to an unexpected modularity breach, as ns translocation truncated the overlapping 3' untranslated region (UTR) of the capsid transcript (vp). We found that the native vp 3' UTR is necessary for high-level VP production but that decreased expression does not adversely impact the expression of NS proteins, which are known replication effectors. As nonsense vp mutations recapitulate the replication defect, VP proteins appear to be directly implicated in the replication process. Our findings suggest intricate replication-encapsidation couplings that favor the maintenance of genetic integrity. We discuss possible connections with an intriguing cis-packaging phenomenon previously observed in parvoviruses whereby capsids preferentially package the genome from which they were expressed. IMPORTANCE Densoviruses could be used as biological control agents to manage insect pests. Such applications require an in-depth biological understanding and associated molecular tools. However, the genomes of these viruses remain difficult to manipulate due to poorly tractable secondary structures at their extremities. We devised a construction strategy that enables precise and efficient molecular modifications. Using this approach, we endeavored to create a split clone of Junonia coenia densovirus (JcDV) that can be used to safely study the impact of capsid mutations on host specificity. Our original construct proved to be nonfunctional. Fixing this defect led us to uncover that capsid proteins and their correct expression are essential for continued rolling-hairpin replication. This points to an intriguing link between replication and packaging, which might be shared with related viruses. This serendipitous discovery illustrates the power of synthetic biology approaches to advance our knowledge of biological systems.
Keywords: capsid; densovirus; replication; synthetic biology.
Figures
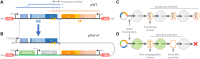





Similar articles
-
Interaction of a Densovirus with Glycans of the Peritrophic Matrix Mediates Oral Infection of the Lepidopteran Pest Spodoptera frugiperda.Viruses. 2019 Sep 17;11(9):870. doi: 10.3390/v11090870. Viruses. 2019. PMID: 31533310 Free PMC article.
-
NS-3 protein of the Junonia coenia densovirus is essential for viral DNA replication in an Ld 652 cell line and Spodoptera littoralis larvae.J Virol. 2004 Jan;78(2):790-7. doi: 10.1128/jvi.78.2.790-797.2004. J Virol. 2004. PMID: 14694111 Free PMC article.
-
Junonia coenia densovirus-based vectors for stable transgene expression in Sf9 cells: influence of the densovirus sequences on genomic integration.J Virol. 2003 Oct;77(20):11060-71. doi: 10.1128/jvi.77.20.11060-11071.2003. J Virol. 2003. PMID: 14512554 Free PMC article.
-
Twenty-Five Years of Structural Parvovirology.Viruses. 2019 Apr 20;11(4):362. doi: 10.3390/v11040362. Viruses. 2019. PMID: 31010002 Free PMC article. Review.
-
The Structures and Functions of Parvovirus Capsids and Missing Pieces: the Viral DNA and Its Packaging, Asymmetrical Features, Nonprotein Components, and Receptor or Antibody Binding and Interactions.J Virol. 2023 Jul 27;97(7):e0016123. doi: 10.1128/jvi.00161-23. Epub 2023 Jun 27. J Virol. 2023. PMID: 37367301 Free PMC article. Review.
Cited by
-
Dynamic integration and evolutionary trajectory of endogenous IHHNV elements in crustacean genomes.DNA Res. 2025 Jul 4;32(4):dsaf018. doi: 10.1093/dnares/dsaf018. DNA Res. 2025. PMID: 40693514 Free PMC article.
-
World Society for Virology first international conference: Tackling global virus epidemics.Virology. 2022 Jan;566:114-121. doi: 10.1016/j.virol.2021.11.009. Epub 2021 Dec 6. Virology. 2022. PMID: 34902730 Free PMC article.
References
-
- Fakhiri J, Schneider MA, Puschhof J, Stanifer M, Schildgen V, Holderbach S, Voss Y, El Andari J, Schildgen O, Boulant S, Meister M, Clevers H, Yan Z, Qiu J, Grimm D. 2019. Novel chimeric gene therapy vectors based on adeno-associated virus and four different mammalian bocaviruses. Mol Ther Methods Clin Dev 12:202–222. 10.1016/j.omtm.2019.01.003. - DOI - PMC - PubMed
-
- Kerr J, Cotmore S, Bloom ME, Linden RM, Parrish CR (ed). 2006. Parvoviruses. Hodder Arnold, London, United Kingdom.
-
- Matthews G. 2018. The spread of fall armyworm (FAW) Spodotera frugiperda. Outlooks Pest Manag 29:213–214. 10.1564/v29_oct_07. - DOI
-
- Bergoin M, Tijssen P. 1998. Biological and molecular properties of densoviruses and their use in protein expression and biological control, p 141–169. In Miller LK, Ball LA (ed), The insect viruses. The viruses. Springer, Boston, MA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

