Arteriopathy in pediatric stroke: an underestimated clinical entity
- PMID: 34133513
- PMCID: PMC9231440
- DOI: 10.1590/0004-282X-ANP-2020-0105
Arteriopathy in pediatric stroke: an underestimated clinical entity
Abstract
Background: Pediatric arterial ischemic stroke (AIS), which was thought to be a rare disorder, is being increasingly recognized as an important cause of neurological morbidity, thanks to new advances in neuroimaging.
Objective: The aim of this study was to review the main etiologies of stroke due to arteriopathy in children.
Methods: Using a series of cases from our institution, we addressed its epidemiological aspects, physiopathology, imaging findings from CT, MR angiography, MR conventional sequences and MR DWI, and nuclear medicine findings.
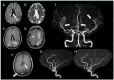
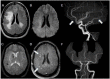
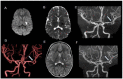
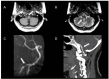
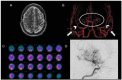
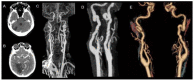
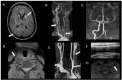
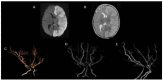
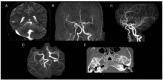
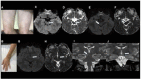
Results: Through discussion of the most recent classification for childhood AIS (Childhood AIS Standardized Classification and Diagnostic Evaluation, CASCADE), we propose a modified classification based on the anatomical site of disease, which includes vasculitis, varicella, arterial dissection, moyamoya, fibromuscular dysplasia, Takayasu's arteritis and genetic causes (such as ACTA-2 mutation, PHACE syndrome and ADA-2 deficiency). We have detailed each of these separately. Conclusions: Prompt recognition of AIS and thorough investigation for potential risk factors are crucial for a better outcome. In this scenario, neurovascular imaging plays an important role in diagnosing AIS and identifying children at high risk of recurrent stroke.
Introdução:: O acidente vascular cerebral (AVC) pediátrico, considerado um distúrbio raro, está sendo cada vez mais reconhecido como importante causa de morbidade neurológica, graças aos novos avanços na neuroimagem.
Objetivo:: Revisar as principais etiologias do AVC por arteriopatia em crianças.
Métodos:: Utilizando-se de uma série de casos de nossa instituição, abordamos seus aspectos epidemiológicos, fisiopatológicos e de imagem na angiotomografia computadorizada e angiorressonância magnética, sequências convencionais e avançadas de ressonância magnética e medicina nuclear.
Resultados:: Com base na classificação mais recente de AVC na infância (Classificação Padronizada e Avaliação Diagnóstica do AVC na Infância - CASCADE) propusemos uma classificação modificada com base no local anatômico da doença, que inclui vasculite, varicela, dissecção arterial, Moyamoya, displasia fibromuscular, arterite de Takayasu e causas genéticas (como mutação ACTA-2, síndrome PHACE e deficiência de ADA-2), detalhando cada uma separadamente.
Conclusões:: O reconhecimento imediato do AVC na infância e a investigação minuciosa de possíveis fatores de risco são cruciais para um melhor resultado. Nesse cenário, a imagem neurovascular desempenha papel importante no diagnóstico de AVC e na identificação de crianças com alto risco de recorrência.
Conflict of interest statement
Figures
References
-
- 1. DeVeber G, Roach ES, Riela AR, Winznizer M. Stroke in children: recognition, treatment, and future directions. Semin Pediatr Neurol. 2000 Dec;7(4):309-17. https://doi.org/10.1053/spen.2000.20074 - PubMed
-
- 2. Ganesan V, Hogan A, Shack N, Gordon A, Isaacs E, Kirkham FJ. Outcome after ischaemic stroke in childhood. Dev Med Child Neurol. 2000 Jul;42(7):455-61. https://doi.org/10.1017/s0012162200000852 - PubMed
-
- 3. Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics. 2007 Mar;119(3):495-501. https://doi.org/10.1542/peds.2006-2791 - PubMed
-
- 4. Fullerton H, Wintermark M, Hills N, Dowling MM, Tan M, Rafay MF, et al. Risk of recurrent arterial ischemic stroke in childhood: a prospective international study. Stroke. 2016 Jan;47(1):53-9. https://doi.org/10.1161/STROKEAHA.115.011173 - PMC - PubMed
-
- 5. Mallick AA, Ganesan V, Kirkham FJ, Fallon P, Hedderly T, McShane T, et al. Childhood arterial ischaemic stroke incidence, presenting features, and risk factors: a prospective population-based study. Lancet Neurol. 2014 Jan;13(1):35-43. https://doi.org/10.1016/S1474-4422(13)70290-4 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
