Chyme Reinfusion Restores the Regulatory Bile Salt-FGF19 Axis in Patients With Intestinal Failure
- PMID: 34133768
- PMCID: PMC8596508
- DOI: 10.1002/hep.32017
Chyme Reinfusion Restores the Regulatory Bile Salt-FGF19 Axis in Patients With Intestinal Failure
Abstract
Background and aims: Automated chyme reinfusion (CR) in patients with intestinal failure (IF) and a temporary double enterostomy (TDE) restores intestinal function and protects against liver injury, but the mechanisms are incompletely understood. The aim was to investigate whether the beneficial effects of CR relate to functional recovery of enterohepatic signaling through the bile salt-FGF19 axis.
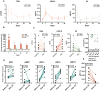
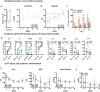
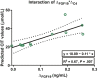
Approach and results: Blood samples were collected from 12 patients, 3 days before, at start, and 1, 3, 5, and 7 weeks after CR initiation. Plasma FGF19, total bile salts (TBS), 7-α-hydroxy-4-cholesten-3-one (C4; a marker of bile salt synthesis), citrulline (CIT), bile salt composition, liver tests, and nutritional risk indices were determined. Paired small bowel biopsies prior to CR and after 21 days were taken, and genes related to bile salt homeostasis and enterocyte function were assessed. CR induced an increase in plasma FGF19 and decreased C4 levels, indicating restored regulation of bile salt synthesis through endocrine FGF19 action. TBS remained unaltered during CR. Intestinal farnesoid X receptor was up-regulated after 21 days of CR. Secondary and deconjugated bile salt fractions were increased after CR, reflecting restored microbial metabolism of host bile salts. Furthermore, CIT and albumin levels gradually rose after CR, while abnormal serum liver tests normalized after CR, indicating restored intestinal function, improved nutritional status, and amelioration of liver injury. CR increased gene transcripts related to enterocyte number, carbohydrate handling, and bile salt homeostasis. Finally, the reciprocal FGF19/C4 response after 7 days predicted the plasma CIT time course.
Conclusions: CR in patients with IF-TDE restored bile salt-FGF19 signaling and improved gut-liver function. Beneficial effects of CR are partly mediated by recovery of the bile salt-FGF19 axis and subsequent homeostatic regulation of bile salt synthesis.
© 2021 The Authors. Hepatology published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Figures



References
-
- Pironi L, Arends J, Baxter J, Bozzetti F, Peláez RB, Cuerda C, et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr 2015;34:171‐180. - PubMed
-
- Oterdoom LH, ten Dam SM, de Groot SDW, Arjaans W, van Bodegraven AA, et al. Limited long‐term survival after in‐hospital intestinal failure requiring total parenteral nutrition. Am J Clin Nutr 2014;100:1102‐1107. - PubMed
-
- Burden S, Hemstock M, Taylor M, Teubner A, Roskell N, MacCulloch A, et al. The impact of home parenteral nutrition on the burden of disease including morbidity, mortality and rate of hospitalisations. Clin Nutr ESPEN 2018;28:222‐227. - PubMed
-
- Rinsema W, Gouma DJ, von Meyenfeldt MF, Soeters PB. Reinfusion of secretions from high‐output proximal stomas or fistulas. Surg Gynecol Obstet 1988;167:372‐376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

