Treatment of Multisystem Inflammatory Syndrome in Children
- PMID: 34133854
- PMCID: PMC8220965
- DOI: 10.1056/NEJMoa2102968
Treatment of Multisystem Inflammatory Syndrome in Children
Abstract
Background: Evidence is urgently needed to support treatment decisions for children with multisystem inflammatory syndrome (MIS-C) associated with severe acute respiratory syndrome coronavirus 2.
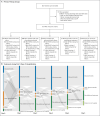
Methods: We performed an international observational cohort study of clinical and outcome data regarding suspected MIS-C that had been uploaded by physicians onto a Web-based database. We used inverse-probability weighting and generalized linear models to evaluate intravenous immune globulin (IVIG) as a reference, as compared with IVIG plus glucocorticoids and glucocorticoids alone. There were two primary outcomes: the first was a composite of inotropic support or mechanical ventilation by day 2 or later or death; the second was a reduction in disease severity on an ordinal scale by day 2. Secondary outcomes included treatment escalation and the time until a reduction in organ failure and inflammation.
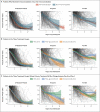
Results: Data were available regarding the course of treatment for 614 children from 32 countries from June 2020 through February 2021; 490 met the World Health Organization criteria for MIS-C. Of the 614 children with suspected MIS-C, 246 received primary treatment with IVIG alone, 208 with IVIG plus glucocorticoids, and 99 with glucocorticoids alone; 22 children received other treatment combinations, including biologic agents, and 39 received no immunomodulatory therapy. Receipt of inotropic or ventilatory support or death occurred in 56 patients who received IVIG plus glucocorticoids (adjusted odds ratio for the comparison with IVIG alone, 0.77; 95% confidence interval [CI], 0.33 to 1.82) and in 17 patients who received glucocorticoids alone (adjusted odds ratio, 0.54; 95% CI, 0.22 to 1.33). The adjusted odds ratios for a reduction in disease severity were similar in the two groups, as compared with IVIG alone (0.90 for IVIG plus glucocorticoids and 0.93 for glucocorticoids alone). The time until a reduction in disease severity was similar in the three groups.
Conclusions: We found no evidence that recovery from MIS-C differed after primary treatment with IVIG alone, IVIG plus glucocorticoids, or glucocorticoids alone, although significant differences may emerge as more data accrue. (Funded by the European Union's Horizon 2020 Program and others; BATS ISRCTN number, ISRCTN69546370.).
Copyright © 2021 Massachusetts Medical Society.
Figures

Comment in
-
Immunotherapy for MIS-C - IVIG, Glucocorticoids, and Biologics.N Engl J Med. 2021 Jul 1;385(1):74-75. doi: 10.1056/NEJMe2108276. Epub 2021 Jun 16. N Engl J Med. 2021. PMID: 34133878 Free PMC article. No abstract available.
-
Therapy for Multisystem Inflammatory Syndrome in Children.N Engl J Med. 2021 Sep 23;385(13):e42. doi: 10.1056/NEJMc2111096. Epub 2021 Aug 11. N Engl J Med. 2021. PMID: 34379918 No abstract available.
-
Therapy for Multisystem Inflammatory Syndrome in Children.N Engl J Med. 2021 Sep 23;385(13):e42. doi: 10.1056/NEJMc2111096. Epub 2021 Aug 11. N Engl J Med. 2021. PMID: 34379919 No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
