Low-dose Ad26.COV2.S protection against SARS-CoV-2 challenge in rhesus macaques
- PMID: 34133941
- PMCID: PMC8166510
- DOI: 10.1016/j.cell.2021.05.040
Low-dose Ad26.COV2.S protection against SARS-CoV-2 challenge in rhesus macaques
Abstract
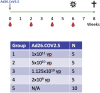
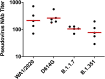
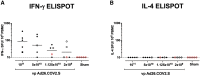
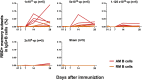
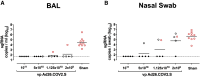
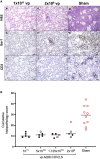
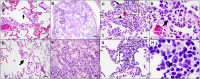
We previously reported that a single immunization with an adenovirus serotype 26 (Ad26)-vector-based vaccine expressing an optimized SARS-CoV-2 spike (Ad26.COV2.S) protected rhesus macaques against SARS-CoV-2 challenge. To evaluate reduced doses of Ad26.COV2.S, 30 rhesus macaques were immunized once with 1 × 1011, 5 × 1010, 1.125 × 1010, or 2 × 109 viral particles (vp) Ad26.COV2.S or sham and were challenged with SARS-CoV-2. Vaccine doses as low as 2 × 109 vp provided robust protection in bronchoalveolar lavage, whereas doses of 1.125 × 1010 vp were required for protection in nasal swabs. Activated memory B cells and binding or neutralizing antibody titers following vaccination correlated with protective efficacy. At suboptimal vaccine doses, viral breakthrough was observed but did not show enhancement of disease. These data demonstrate that a single immunization with relatively low dose of Ad26.COV2.S effectively protected against SARS-CoV-2 challenge in rhesus macaques, although a higher vaccine dose may be required for protection in the upper respiratory tract.
Keywords: Ad26.COV2.S; COVID-19; SARS-CoV-2; humoral immunity; immunology; memory B cells; non-human primates; protection; vaccination.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.H.B., R.Z., F.W., and H.S are co-inventors on provisional vaccine patents (63/121,482; 63/133,969; 63/135,182). R.Z., F.W., S.R.H., M.v.H., L.v.d.F., and H.S. are employees of Janssen Vaccines & Prevention BV and may hold stock in Johnson & Johnson.
Figures







Update of
-
Low-Dose Ad26.COV2.S Protection Against SARS-CoV-2 Challenge in Rhesus Macaques.bioRxiv [Preprint]. 2021 Jan 27:2021.01.27.428380. doi: 10.1101/2021.01.27.428380. bioRxiv. 2021. Update in: Cell. 2021 Jun 24;184(13):3467-3473.e11. doi: 10.1016/j.cell.2021.05.040. PMID: 33532782 Free PMC article. Updated. Preprint.
References
-
- Abbink P., Lemckert A.A., Ewald B.A., Lynch D.M., Denholtz M., Smits S., Holterman L., Damen I., Vogels R., Thorner A.R., et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 2007;81:4654–4663. - PMC - PubMed
-
- Bos R., Rutten L., van der Lubbe J.E.M., Bakkers M.J.G., Hardenberg G., Wegmann F., Zuijdgeest D., de Wilde A.H., Koornneef A., Verwilligen A., et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020;5:91. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

