Accuracy of COVID-19 rapid antigenic tests compared to RT-PCR in a student population: The StudyCov study
- PMID: 34134035
- PMCID: PMC8178956
- DOI: 10.1016/j.jcv.2021.104878
Accuracy of COVID-19 rapid antigenic tests compared to RT-PCR in a student population: The StudyCov study
Abstract
Objective: There is a lack of data evaluating performance of antigenic test (AT) for SARS-CoV-2 diagnosis (Ag-RDT) in clinical practice, especially in asymptomatic subjects. The main objective of this study was to evaluate the diagnostic performance of AT compared to Reverse Transcription Polymerase Chain Reaction (RT-PCR) for SARS-CoV-2 diagnosis.
Methods: StudyCov is a monocentric cross-sectional study. A SARS-CoV-2 screening facility was set up in the Bordeaux University health campus from October 28th to November 20th 2020. Students willing to have a RT-PCR test (ARGENE SARS-CoV-2 R-GENE, BioMérieux, France) for SARS-CoV-2 diagnosis were also offered the Abbott Panbio™ SARS-CoV-2 antigenic rapid test. All participants attending the screening facility with an AT in addition to RT-PCR and having signed an informed consent were included in the study. The main objective was to assess performance of AT as compared with RT-PCR in the recruited population. Secondary objectives dealt with the analysis of the main objective stratified by current symptoms and risk exposure. A sensitivity analysis with different RT-PCR cycle thresholds was included.
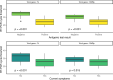
Results: RT-PCR and AT results were available for 692 subjects. Overall sensitivity and specificity of AT tests were respectively 63.5% (95% confidence interval (CI): 49.0 - 76.4) and 100% (95% CI: 99.4 - 100). In the asymptomatic sub-group, they were respectively 35.0% (95% CI: 15.4% - 59.2%) and 100% (95% CI: 99.3 - 100).
Conclusions: This study shows the poor sensitivity of AT in asymptomatic subjects, specificity being however excellent. The performance results fall below the World Health Organization recommendation of 80% sensitivity and question using AT in general population, especially when asymptomatic.
Keywords: COVID19; Point-of-care testing; Rapid antigen detection test; Rt-pcr; Sars-cov-2.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Canada PHA of Interim guidance on the use of rapid antigen detection tests for the identification of SARS-CoV-2 infection. Aem. 2020 https://www.canada.ca/en/public-health/services/diseases/2019-novel-coro... (accessed December 9, 2020)
-
- CDC Information for Laboratories about Coronavirus (COVID-19) Cent Dis Control Prev. 2020 https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-gu... (accessed December 9, 2020)
-
- World Health Organisation. Antigen-detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays (2020). https://www.who.int/publications-detail-redirect/antigen-detection-in-th... (accessed December 9, 2020).
-
- Autorité de Santé Haute. Revue rapide sur les tests de détection antigénique du virus SARS-CoV-2. 2020:42.
-
- European Center for Disease Prevention and Control, Options for the Use of Rapid Antigen Tests For COVID-19 in the EU/EEA and the UK (2020), Tech Rep n.d.:21.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous