Reductions in endometriosis-associated pain among women treated with elagolix are consistent across a range of baseline characteristics reflective of real-world patients
- PMID: 34134684
- PMCID: PMC8210385
- DOI: 10.1186/s12905-021-01385-3
Reductions in endometriosis-associated pain among women treated with elagolix are consistent across a range of baseline characteristics reflective of real-world patients
Abstract
Background: Elagolix is an oral, gonadotropin-releasing hormone (GnRH) receptor antagonist, that significantly reduces dysmenorrhea and non-menstrual pelvic pain (NMPP) in women with moderate to severe endometriosis-associated pain.
Methods: Data were pooled from two 6-month, placebo-controlled, phase 3 studies (Elaris Endometriosis [EM]-I and II) in which 2 doses of elagolix were evaluated (150 mg once daily and 200 mg twice daily). Pooled data from > 1600 women, aged 18-49, were used to evaluate the efficacy of elagolix and health-related quality of life (HRQoL) in prespecified subgroups of women with various baseline characteristics.
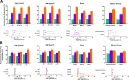
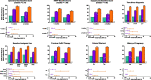
Results: Of the 1686 women treated, 1285 (76.2%) completed the studies. The percentages of women with clinically meaningful reductions in dysmenorrhea and NMPP were generally consistent by subgroup. Significant treatment by subgroup interaction was demonstrated for dysmenorrhea response in baseline analgesic use (p < 0.01) and previous history of pregnancy (p < 0.05) subgroups, and for NMPP response in the baseline NMPP score (p < 0.05) and history of pregnancy (p < 0.05) subgroups. Patient-reported reduction in pain at month 3 was significant across all subgroups taking elagolix 200 mg BID, and significant across most subgroups with elagolix 150 mg QD. Women across subgroups experienced improvement within each domain of the Endometriosis Health Profile-30 (EHP-30), although significant treatment by subgroup interactions were observed in several categories.
Conclusions: Elagolix was effective in reducing dysmenorrhea and NMPP, and improving HRQoL, compared with placebo across numerous subgroups of women with various baseline characteristics, covering a broad segment of the endometriosis disease and patient types.
Clinical trial registration: ClinicalTrials.gov: https://www.clinicaltrials.gov/ct2/show/NCT01620528 ; https://www.clinicaltrials.gov/ct2/show/NCT01931670 .
Keywords: Dysmenorrhea; Dyspareunia; Endometriosis; Gonadotropin-releasing hormone; Health-related quality of life.
Conflict of interest statement
MSA reports roles as Vice President of the American Association of Gynecologic Laparoscopists and Editor-in-Chief of the
Figures

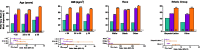
References
-
- Lagana AS, Salmeri FM, Ban Frangez H, Ghezzi F, Vrtacnik-Bokal E, Granese R. Evaluation of M1 and M2 macrophages in ovarian endometriomas from women affected by endometriosis at different stages of the disease. Gynecol Endocrinol. 2020;36(5):441–444. doi: 10.1080/09513590.2019.1683821. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

