Cyclic AMP Response Element Modulator-α Suppresses PD-1 Expression and Promotes Effector CD4+ T Cells in Psoriasis
- PMID: 34135066
- PMCID: PMC8674367
- DOI: 10.4049/jimmunol.2100240
Cyclic AMP Response Element Modulator-α Suppresses PD-1 Expression and Promotes Effector CD4+ T Cells in Psoriasis
Abstract
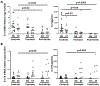
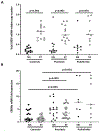
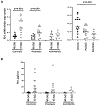
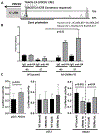
Effector CD4+ T lymphocytes contribute to inflammation and tissue damage in psoriasis, but the underlying molecular mechanisms remain poorly understood. The transcription factor CREMα controls effector T cell function in people with systemic autoimmune diseases. The inhibitory surface coreceptor PD-1 plays a key role in the control of effector T cell function and its therapeutic inhibition in patients with cancer can cause psoriasis. In this study, we show that CD4+ T cells from patients with psoriasis and psoriatic arthritis exhibit increased production of IL-17 but decreased expression of IL-2 and PD-1. In genetically modified mice and Jurkat T cells CREMα expression was linked to low PD-1 levels. We demonstrate that CREMα is recruited to the proximal promoter of PDCD1 in which it trans-represses gene expression and corecruits DNMT3a-mediating DNA methylation. As keratinocytes limit inflammation by PD-1 ligand expression and, in this study, reported reduced expression of PD-1 on CD4+ T cells is linked to low IL-2 and high IL-17A production, our studies reveal a molecular pathway in T cells from people with psoriasis that can deserve clinical exploitation.
Copyright © 2021 by The American Association of Immunologists, Inc.
Figures




Similar articles
-
cAMP response element modulator α controls IL2 and IL17A expression during CD4 lineage commitment and subset distribution in lupus.Proc Natl Acad Sci U S A. 2012 Oct 9;109(41):16606-11. doi: 10.1073/pnas.1210129109. Epub 2012 Sep 26. Proc Natl Acad Sci U S A. 2012. PMID: 23019580 Free PMC article.
-
cAMP Response Element Modulator α Induces Dual Specificity Protein Phosphatase 4 to Promote Effector T Cells in Juvenile-Onset Lupus.J Immunol. 2019 Dec 1;203(11):2807-2816. doi: 10.4049/jimmunol.1900760. Epub 2019 Oct 25. J Immunol. 2019. PMID: 31653682 Free PMC article.
-
Increased Set1 binding at the promoter induces aberrant epigenetic alterations and up-regulates cyclic adenosine 5'-monophosphate response element modulator alpha in systemic lupus erythematosus.Clin Epigenetics. 2016 Nov 24;8:126. doi: 10.1186/s13148-016-0294-2. eCollection 2016. Clin Epigenetics. 2016. PMID: 27904655 Free PMC article.
-
cAMP responsive element modulator α promotes effector T cells in systemic autoimmune diseases.Immunology. 2023 Dec;170(4):470-482. doi: 10.1111/imm.13680. Epub 2023 Jul 12. Immunology. 2023. PMID: 37435993 Review.
-
The cAMP responsive element modulator (CREM) is a regulator of CD4+ T cell function.Biol Chem. 2021 Aug 26;402(12):1591-1596. doi: 10.1515/hsz-2021-0249. Print 2021 Nov 25. Biol Chem. 2021. PMID: 34448385 Review.
Cited by
-
Histone and Histone Acetylation-Related Alterations of Gene Expression in Uninvolved Psoriatic Skin and Their Effects on Cell Proliferation, Differentiation, and Immune Responses.Int J Mol Sci. 2023 Sep 26;24(19):14551. doi: 10.3390/ijms241914551. Int J Mol Sci. 2023. PMID: 37833997 Free PMC article.
-
DNA methylation patterns in CD4+ T-cells separate psoriasis patients from healthy controls, and skin psoriasis from psoriatic arthritis.Front Immunol. 2023 Aug 15;14:1245876. doi: 10.3389/fimmu.2023.1245876. eCollection 2023. Front Immunol. 2023. PMID: 37662940 Free PMC article.
References
-
- Brandt D, Sergon M, Abraham S, Mabert K, and Hedrich CM. TCR(+)CD3(+)CD4(−)CD8(−) effector T cells in psoriasis. Clin Immunol. 2017;181:51–59. - PubMed
-
- Krueger JG, Fretzin S, Suarez-Farinas M, Haslett PA, Phipps KM, Cameron GS, McColm J, Katcherian A, Cueto I, White T, Banerjee S, and Hoffman RW. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol. 2012;130(1):145–154 e149. - PMC - PubMed
-
- Sallusto F, Lenig D, Forster R, Lipp M, and Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

