Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients
- PMID: 34135083
- PMCID: PMC8729854
- DOI: 10.1681/ASN.2021040490
Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients
Abstract
Background: Kidney transplant recipients and patients receiving hemodialysis are immunocompromised populations that are prioritized for COVID-19 vaccination but were excluded from clinical trials of SARS-CoV-2 mRNA vaccines. Antibody titers and rates of seroconversion after vaccination are lower among patients with CKD and those taking immunosuppressants compared with controls. Data are lacking regarding their humoral response to vaccination to prevent COVID-19.
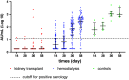
Methods: This investigation of early serological response after COVID-19 vaccination with the Pfizer/BioNTech (BNT162b2) mRNA vaccine included 78 patients undergoing hemodialysis, 74 kidney transplant recipients, and seven healthy controls. We recorded data from the medical file for various clinical parameters, including response to hepatitis B vaccination, and measured antibody titers against SARS-CoV-2 at 0, 14, 28, 36, and 58 days after the first injection.
Results: In controls, we detected antibodies at a positive level (>13 arbitrary units per ml; AU/ml) at day 14 postinjection, which increased progressively to peak at day 36 (1082 AU/ml; interquartile range [IQR], 735.0-1662.0). Patients undergoing hemodialysis had lower titers that peaked at day 58 (276 AU/ml; IQR, 83.4-526.0). We detected a positive antibody level in only three transplant recipients at day 36. In patients on hemodialysis, those aged <75 years had a higher antibody response versus those aged >75 years, and serum albumin and Kt/V were positively correlated with serological response (P<0.04 and P<0.0, respectively); nonresponders to HBV vaccine had the lowest anti-SARS-CoV-2 antibody titers.
Conclusions: Our results suggest that the postvaccination humoral response is strongly inhibited by immunosuppressant therapy in kidney transplant recipients, and is reduced by the uremic condition in patients undergoing hemodialysis.
Keywords: COVID-19; hemodialyzed; humoral response; kidney transplant recipients; kinetic; vaccine.
Copyright © 2021 by the American Society of Nephrology.
Figures

References
-
- Kronbichler A, Anders HJ, Fernandez-Juárez GM, Floege J, Goumenos D, Segelmark M, et al. .; Immunonephrology Working Group of the ERA-EDTA (European Renal Association – European Dialysis, Transplant Association): Recommendations for the use of COVID-19 vaccines in patients with immune-mediated kidney diseases [published online ahead of print March 9, 2021]. Nephrol Dial Transplant - PMC - PubMed
-
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. .: Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586: 589–593, 2020 - PubMed
-
- Krueger KM, Ison MG, Ghossein C: Practical guide to vaccination in all stages of CKD, including patients treated by dialysis or kidney transplantation. Am J Kidney Dis 75: 417–425, 2020 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

