Mice Expressing Regulators of G protein Signaling-insensitive Gαo Define Roles of μ Opioid Receptor G α o and G α i Subunit Coupling in Inhibition of Presynaptic GABA Release
- PMID: 34135098
- PMCID: PMC8626785
- DOI: 10.1124/molpharm.121.000249
Mice Expressing Regulators of G protein Signaling-insensitive Gαo Define Roles of μ Opioid Receptor G α o and G α i Subunit Coupling in Inhibition of Presynaptic GABA Release
Abstract
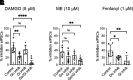
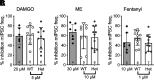
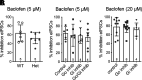
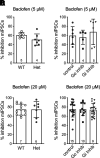
Regulators of G protein signaling (RGS) proteins modulate signaling by G protein-coupled receptors. Using a knock-in transgenic mouse model with a mutation in Gαo that does not bind RGS proteins (RGS-insensitive), we determined the effect of RGS proteins on presynaptic μ opioid receptor (MOR)-mediated inhibition of GABA release in the ventrolateral periaqueductal gray (vlPAG). The MOR agonists [d-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) and met-enkephalin (ME) inhibited evoked inhibitory postsynaptic currents (eIPSCs) in the RGS-insensitive mice compared with wild-type (WT) littermates, respectively. Fentanyl inhibited eIPSCs similarly in both WT and RGS-insensitive mice. There were no differences in opioid agonist inhibition of spontaneous GABA release between the genotypes. To further probe the mechanism underlying these differences between opioid inhibition of evoked and spontaneous GABA release, specific myristoylated Gα peptide inhibitors for Gαo1 and Gαi1-3 that block receptor-G protein interactions were used to test the preference of agonists for MOR-Gα complexes. The Gαo1 inhibitor reduced DAMGO inhibition of eIPSCs, but Gαi1-3 inhibitors had no effect. Both Gαo1 and Gαi1-3 inhibitors separately reduced fentanyl inhibition of eIPSCs but had no effects on ME inhibition. Gαi1-3 inhibitors blocked the inhibitory effects of ME and fentanyl on miniature postsynaptic current (mIPSC) frequency, but both Gαo1 and Gαi1-3 inhibitors were needed to block the effects of DAMGO. Finally, baclofen-mediated inhibition of GABA release is unaffected in the RGS-insensitive mice and in the presence of Gαo1 and Gαi1-3 inhibitor peptides, suggesting that GABAB receptor coupling to G proteins in vlPAG presynaptic terminals is different than MOR coupling. SIGNIFICANCE STATEMENT: Presynaptic μ opioid receptors (MORs) in the ventrolateral periaqueductal gray are critical for opioid analgesia and are negatively regulated by RGS proteins. These data in RGS-insensitive mice provide evidence that MOR agonists differ in preference for Gαo versus Gαi and regulation by RGS proteins in presynaptic terminals, providing a mechanism for functional selectivity between agonists. The results further define important differences in MOR and GABAB receptor coupling to G proteins that could be exploited for new pain therapies.
Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
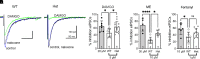

Similar articles
-
Regulators of G-Protein Signaling (RGS) Proteins Promote Receptor Coupling to G-Protein-Coupled Inwardly Rectifying Potassium (GIRK) Channels.J Neurosci. 2018 Oct 10;38(41):8737-8744. doi: 10.1523/JNEUROSCI.0516-18.2018. Epub 2018 Aug 27. J Neurosci. 2018. PMID: 30150362 Free PMC article.
-
Differential control of opioid antinociception to thermal stimuli in a knock-in mouse expressing regulator of G-protein signaling-insensitive Gαo protein.J Neurosci. 2013 Mar 6;33(10):4369-77. doi: 10.1523/JNEUROSCI.5470-12.2013. J Neurosci. 2013. PMID: 23467353 Free PMC article.
-
Mu Receptors.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31855381 Free Books & Documents.
-
Effector antagonism by the regulators of G protein signalling (RGS) proteins causes desensitization of mu-opioid receptors in the CNS.Psychopharmacology (Berl). 2005 Jun;180(1):1-11. doi: 10.1007/s00213-005-2248-9. Epub 2005 Apr 14. Psychopharmacology (Berl). 2005. PMID: 15830230 Review.
-
RGS-Insensitive G Proteins as In Vivo Probes of RGS Function.Prog Mol Biol Transl Sci. 2015;133:13-30. doi: 10.1016/bs.pmbts.2015.04.010. Epub 2015 Jun 6. Prog Mol Biol Transl Sci. 2015. PMID: 26123300 Review.
Cited by
-
Fine-tuning GPCR-mediated neuromodulation by biasing signaling through different G protein subunits.Mol Cell. 2023 Jul 20;83(14):2540-2558.e12. doi: 10.1016/j.molcel.2023.06.006. Epub 2023 Jun 29. Mol Cell. 2023. PMID: 37390816 Free PMC article.
-
Delta opioid receptors engage multiple signaling cascades to differentially modulate prefrontal GABA release with input and target specificity.bioRxiv [Preprint]. 2024 Aug 9:2024.08.08.607246. doi: 10.1101/2024.08.08.607246. bioRxiv. 2024. Update in: Cell Rep. 2025 Feb 25;44(2):115293. doi: 10.1016/j.celrep.2025.115293. PMID: 39149233 Free PMC article. Updated. Preprint.
-
Cellular and circuit diversity determines the impact of endogenous opioids in the descending pain modulatory pathway.Front Syst Neurosci. 2022 Aug 15;16:963812. doi: 10.3389/fnsys.2022.963812. eCollection 2022. Front Syst Neurosci. 2022. PMID: 36045708 Free PMC article. Review.
-
Monoacylglycerol Lipase Protects the Presynaptic Cannabinoid 1 Receptor from Desensitization by Endocannabinoids after Persistent Inflammation.J Neurosci. 2023 Jul 26;43(30):5458-5467. doi: 10.1523/JNEUROSCI.0037-23.2023. Epub 2023 Jul 6. J Neurosci. 2023. PMID: 37414560 Free PMC article.
References
-
- Budai D, Fields HL (1998) Endogenous opioid peptides acting at mu-opioid receptors in the dorsal horn contribute to midbrain modulation of spinal nociceptive neurons. J Neurophysiol 79:677–687. - PubMed
-
- Chakrabarti S, Prather PL, Yu L, Law PY, Loh HH (1995) Expression of the mu-opioid receptor in CHO cells: ability of mu-opioid ligands to promote alpha-azidoanilido[32P]GTP labeling of multiple G protein alpha subunits. J Neurochem 64:2534–2543. - PubMed
-
- Cheng ZF, Fields HL, Heinricher MM (1986) Morphine microinjected into the periaqueductal gray has differential effects on 3 classes of medullary neurons. Brain Res 375:57–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

