Dissection-independent production of Plasmodium sporozoites from whole mosquitoes
- PMID: 34135099
- PMCID: PMC8321652
- DOI: 10.26508/lsa.202101094
Dissection-independent production of Plasmodium sporozoites from whole mosquitoes
Abstract
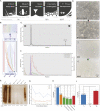
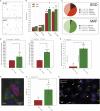
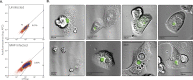
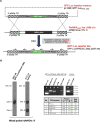
Progress towards a protective vaccine against malaria remains slow. To date, only limited protection has been routinely achieved following immunisation with either whole-parasite (sporozoite) or subunit-based vaccines. One major roadblock to vaccine progress, and to pre-erythrocytic parasite biology in general, is the continued reliance on manual salivary gland dissection for sporozoite isolation from infected mosquitoes. Here, we report development of a multi-step method, based on batch processing of homogenised whole mosquitoes, slurry, and density-gradient filtration, which combined with free-flow electrophoresis rapidly produces a pure, infective sporozoite inoculum. Human-infective Plasmodium falciparum and rodent-infective Plasmodium berghei sporozoites produced in this way are two- to threefold more infective than salivary gland dissection sporozoites in in vitro hepatocyte infection assays. In an in vivo rodent malaria model, the same P. berghei sporozoites confer sterile protection from mosquito-bite challenge when immunisation is delivered intravenously or 60-70% protection when delivered intramuscularly. By improving purity, infectivity, and immunogenicity, this method represents a key advancement in capacity to produce research-grade sporozoites, which should impact delivery of a whole-parasite based malaria vaccine at scale in the future.
© 2021 Blight et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







References
-
- WHO (2019) World Malaria Report. Geneva: Switzerland: WHO.
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
