Copper-binding anticancer peptides from the piscidin family: an expanded mechanism that encompasses physical and chemical bilayer disruption
- PMID: 34135370
- PMCID: PMC8208971
- DOI: 10.1038/s41598-021-91670-w
Copper-binding anticancer peptides from the piscidin family: an expanded mechanism that encompasses physical and chemical bilayer disruption
Abstract
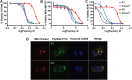
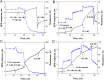
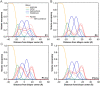
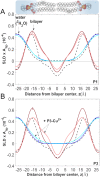
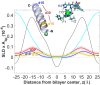
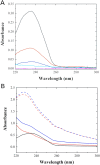
In the search for novel broad-spectrum therapeutics to fight chronic infections, inflammation, and cancer, host defense peptides (HDPs) have garnered increasing interest. Characterizing their biologically-active conformations and minimum motifs for function represents a requisite step to developing them into efficacious and safe therapeutics. Here, we demonstrate that metallating HDPs with Cu2+ is an effective chemical strategy to improve their cytotoxicity on cancer cells. Mechanistically, we find that prepared as Cu2+-complexes, the peptides not only physically but also chemically damage lipid membranes. Our testing ground features piscidins 1 and 3 (P1/3), two amphipathic, histidine-rich, membrane-interacting, and cell-penetrating HDPs that are α-helical bound to membranes. To investigate their membrane location, permeabilization effects, and lipid-oxidation capability, we employ neutron reflectometry, impedance spectroscopy, neutron diffraction, and UV spectroscopy. While P1-apo is more potent than P3-apo, metallation boosts their cytotoxicities by up to two- and seven-fold, respectively. Remarkably, P3-Cu2+ is particularly effective at inserting in bilayers, causing water crevices in the hydrocarbon region and placing Cu2+ near the double bonds of the acyl chains, as needed to oxidize them. This study points at a new paradigm where complexing HDPs with Cu2+ to expand their mechanistic reach could be explored to design more potent peptide-based anticancer therapeutics.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Enhancing the membrane activity of Piscidin 1 through peptide metallation and the presence of oxidized lipid species: Implications for the unification of host defense mechanisms at lipid membranes.Biochim Biophys Acta Biomembr. 2020 Jul 1;1862(7):183236. doi: 10.1016/j.bbamem.2020.183236. Epub 2020 Feb 29. Biochim Biophys Acta Biomembr. 2020. PMID: 32126226
-
Nuclease activity gives an edge to host-defense peptide piscidin 3 over piscidin 1, rendering it more effective against persisters and biofilms.FEBS J. 2017 Nov;284(21):3662-3683. doi: 10.1111/febs.14263. Epub 2017 Sep 30. FEBS J. 2017. PMID: 28892294 Free PMC article.
-
Copper regulates the interactions of antimicrobial piscidin peptides from fish mast cells with formyl peptide receptors and heparin.J Biol Chem. 2018 Oct 5;293(40):15381-15396. doi: 10.1074/jbc.RA118.001904. Epub 2018 Aug 29. J Biol Chem. 2018. PMID: 30158246 Free PMC article.
-
Solution NMR studies of cell-penetrating peptides in model membrane systems.Adv Drug Deliv Rev. 2013 Jul;65(8):1002-11. doi: 10.1016/j.addr.2012.10.011. Epub 2012 Nov 6. Adv Drug Deliv Rev. 2013. PMID: 23137785 Review.
-
Membrane-active peptides: binding, translocation, and flux in lipid vesicles.Biochim Biophys Acta. 2014 Sep;1838(9):2216-27. doi: 10.1016/j.bbamem.2014.04.014. Epub 2014 Apr 25. Biochim Biophys Acta. 2014. PMID: 24769436 Free PMC article. Review.
Cited by
-
Antiviral activity of the host defense peptide piscidin 1: investigating a membrane-mediated mode of action.Front Chem. 2024 Jun 26;12:1379192. doi: 10.3389/fchem.2024.1379192. eCollection 2024. Front Chem. 2024. PMID: 38988727 Free PMC article.
-
Host-defense piscidin peptides as antibiotic adjuvants against Clostridioides difficile.PLoS One. 2024 Jan 22;19(1):e0295627. doi: 10.1371/journal.pone.0295627. eCollection 2024. PLoS One. 2024. PMID: 38252641 Free PMC article.
-
Investigating How Lysophosphatidylcholine and Lysophosphatidylethanolamine Enhance the Membrane Permeabilization Efficacy of Host Defense Peptide Piscidin 1.J Phys Chem B. 2025 Jan 9;129(1):210-227. doi: 10.1021/acs.jpcb.4c05845. Epub 2024 Dec 16. J Phys Chem B. 2025. PMID: 39681296
-
Synergistic Treatment of Breast Cancer by Combining the Antimicrobial Peptide Piscidin with a Modified Glycolipid.ACS Omega. 2024 Jul 24;9(31):33408-33424. doi: 10.1021/acsomega.3c09902. eCollection 2024 Aug 6. ACS Omega. 2024. PMID: 39130564 Free PMC article.
-
Anticancer Activity of Metallodrugs and Metallizing Host Defense Peptides-Current Developments in Structure-Activity Relationship.Int J Mol Sci. 2024 Jul 3;25(13):7314. doi: 10.3390/ijms25137314. Int J Mol Sci. 2024. PMID: 39000421 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

