Assessment with clinical data of a coupled bio-hemodynamics numerical model to predict leukocyte adhesion in coronary arteries
- PMID: 34135399
- PMCID: PMC8208986
- DOI: 10.1038/s41598-021-92084-4
Assessment with clinical data of a coupled bio-hemodynamics numerical model to predict leukocyte adhesion in coronary arteries
Abstract
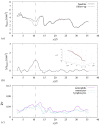
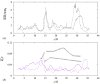
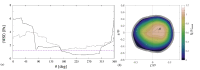
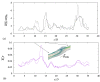
Numerical simulations of coupled hemodynamics and leukocyte transport and adhesion inside coronary arteries have been performed. Realistic artery geometries have been obtained for a set of four patients from intravascular ultrasound and angiography images. The numerical model computes unsteady three-dimensional blood hemodynamics and leukocyte concentration in the blood. Wall-shear stress dependent leukocyte adhesion is also computed through agent-based modeling rules, fully coupled to the hemodynamics and leukocyte transport. Numerical results have a good correlation with clinical data. Regions where high adhesion is predicted by the simulations coincide to a good approximation with artery segments presenting plaque increase, as documented by clinical data from baseline and six-month follow-up exam of the same artery. In addition, it is observed that the artery geometry and, in particular, the tortuosity of the centerline are a primary factor in determining the spatial distribution of wall-shear stress, and of the resulting leukocyte adhesion patterns. Although further work is required to overcome the limitations of the present model and ultimately quantify plaque growth in the simulations, these results are encouraging towards establishing a predictive methodology for atherosclerosis progress.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

