A cohort autopsy study defines COVID-19 systemic pathogenesis
- PMID: 34135479
- PMCID: PMC8208380
- DOI: 10.1038/s41422-021-00523-8
A cohort autopsy study defines COVID-19 systemic pathogenesis
Abstract
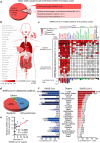
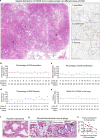
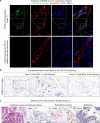
Severe COVID-19 disease caused by SARS-CoV-2 is frequently accompanied by dysfunction of the lungs and extrapulmonary organs. However, the organotropism of SARS-CoV-2 and the port of virus entry for systemic dissemination remain largely unknown. We profiled 26 COVID-19 autopsy cases from four cohorts in Wuhan, China, and determined the systemic distribution of SARS-CoV-2. SARS-CoV-2 was detected in the lungs and multiple extrapulmonary organs of critically ill COVID-19 patients up to 67 days after symptom onset. Based on organotropism and pathological features of the patients, COVID-19 was divided into viral intrapulmonary and systemic subtypes. In patients with systemic viral distribution, SARS-CoV-2 was detected in monocytes, macrophages, and vascular endothelia at blood-air barrier, blood-testis barrier, and filtration barrier. Critically ill patients with long disease duration showed decreased pulmonary cell proliferation, reduced viral RNA, and marked fibrosis in the lungs. Permanent SARS-CoV-2 presence and tissue injuries in the lungs and extrapulmonary organs suggest direct viral invasion as a mechanism of pathogenicity in critically ill patients. SARS-CoV-2 may hijack monocytes, macrophages, and vascular endothelia at physiological barriers as the ports of entry for systemic dissemination. Our study thus delineates systemic pathological features of SARS-CoV-2 infection, which sheds light on the development of novel COVID-19 treatment.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

