The role of regulated necrosis in endocrine diseases
- PMID: 34135504
- PMCID: PMC8207819
- DOI: 10.1038/s41574-021-00499-w
The role of regulated necrosis in endocrine diseases
Abstract
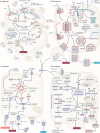
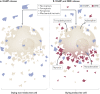
The death of endocrine cells is involved in type 1 diabetes mellitus, autoimmunity, adrenopause and hypogonadotropism. Insights from research on basic cell death have revealed that most pathophysiologically important cell death is necrotic in nature, whereas regular metabolism is maintained by apoptosis programmes. Necrosis is defined as cell death by plasma membrane rupture, which allows the release of damage-associated molecular patterns that trigger an immune response referred to as necroinflammation. Regulated necrosis comes in different forms, such as necroptosis, pyroptosis and ferroptosis. In this Perspective, with a focus on the endocrine environment, we introduce these cell death pathways and discuss the specific consequences of regulated necrosis. Given that clinical trials of necrostatins for the treatment of autoimmune conditions have already been initiated, we highlight the therapeutic potential of such novel therapeutic approaches that, in our opinion, should be tested in endocrine disorders in the future.
© 2021. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

