Geometry of abstract learned knowledge in the hippocampus
- PMID: 34135512
- PMCID: PMC9549979
- DOI: 10.1038/s41586-021-03652-7
Geometry of abstract learned knowledge in the hippocampus
Abstract
Hippocampal neurons encode physical variables1-7 such as space1 or auditory frequency6 in cognitive maps8. In addition, functional magnetic resonance imaging studies in humans have shown that the hippocampus can also encode more abstract, learned variables9-11. However, their integration into existing neural representations of physical variables12,13 is unknown. Here, using two-photon calcium imaging, we show that individual neurons in the dorsal hippocampus jointly encode accumulated evidence with spatial position in mice performing a decision-making task in virtual reality14-16. Nonlinear dimensionality reduction13 showed that population activity was well-described by approximately four to six latent variables, which suggests that neural activity is constrained to a low-dimensional manifold. Within this low-dimensional space, both physical and abstract variables were jointly mapped in an orderly manner, creating a geometric representation that we show is similar across mice. The existence of conjoined cognitive maps suggests that the hippocampus performs a general computation-the creation of task-specific low-dimensional manifolds that contain a geometric representation of learned knowledge.
Figures




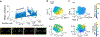
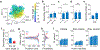







Comment in
-
A conjoined cognitive map.Nat Rev Neurosci. 2021 Sep;22(9):518-519. doi: 10.1038/s41583-021-00506-z. Nat Rev Neurosci. 2021. PMID: 34331038 No abstract available.
References
-
- O’Keefe J. & Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 (1971). - PubMed
-
- Frank LM, Brown EN & Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron 27, 169–178 (2000). - PubMed
-
- Wood ER, Dudchenko PA, Robitsek RJ & Eichenbaum H. Hippocampal Neurons Encode Information about Different Types of Memory Episodes Occurring in the Same Location. Neuron 27, 623–633 (2000). - PubMed
Methods references
-
- Rich PD, Liaw H-P & Lee AK Place cells. Large environments reveal the statistical structure governing hippocampal representations. Science 345, 814–817 (2014). - PubMed
-
- Tenenbaum JB, Silva V. de & Langford JC A Global Geometric Framework for Nonlinear Dimensionality Reduction. Science 290, 2319–2323 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

