Localization atomic force microscopy
- PMID: 34135520
- PMCID: PMC8697813
- DOI: 10.1038/s41586-021-03551-x
Localization atomic force microscopy
Abstract
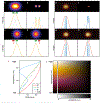
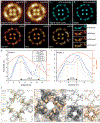
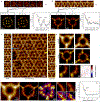
Understanding structural dynamics of biomolecules at the single-molecule level is vital to advancing our knowledge of molecular mechanisms. Currently, there are few techniques that can capture dynamics at the sub-nanometre scale and in physiologically relevant conditions. Atomic force microscopy (AFM)1 has the advantage of analysing unlabelled single molecules in physiological buffer and at ambient temperature and pressure, but its resolution limits the assessment of conformational details of biomolecules2. Here we present localization AFM (LAFM), a technique developed to overcome current resolution limitations. By applying localization image reconstruction algorithms3 to peak positions in high-speed AFM and conventional AFM data, we increase the resolution beyond the limits set by the tip radius, and resolve single amino acid residues on soft protein surfaces in native and dynamic conditions. LAFM enables the calculation of high-resolution maps from either images of many molecules or many images of a single molecule acquired over time, facilitating single-molecule structural analysis. LAFM is a post-acquisition image reconstruction method that can be applied to any biomolecular AFM dataset.
Conflict of interest statement
Competing interests:
The authors declare no competing interests.
Figures










Comment in
-
Atomic force microscopy in super-resolution.Nat Methods. 2021 Aug;18(8):859. doi: 10.1038/s41592-021-01246-9. Nat Methods. 2021. PMID: 34354285 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

