Efficacy and Treatment Strategies in Advanced Cancers with Liver Metastasis Receiving Atezolizumab Therapy
- PMID: 34135631
- PMCID: PMC8197666
- DOI: 10.2147/CMAR.S310331
Efficacy and Treatment Strategies in Advanced Cancers with Liver Metastasis Receiving Atezolizumab Therapy
Abstract
Background: Atezolizumab has been used to treat patients with liver metastasis (LM). However, whether atezolizumab is superior to standard of care therapy in an all-comer or selective population with LM is still uncertain.
Methods: A pooled analysis based on 10 randomized controlled trials was conducted to evaluate the clinical benefit of atezolizumab versus standard therapy in patients stratified by liver metastatic status, followed by biomarker-based individual analyses of the non-small cell lung cancer (NSCLC) cohort (OAK and POPLAR studies) and urothelial cancer cohort (IMvigor210 study).
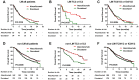
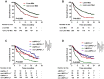
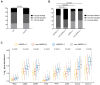
Results: The pooled analysis demonstrated an overall survival (OS) improvement using atezolizumab treatment versus standard therapy across cancer types and treatment lines regardless of liver metastatic status. However, the efficacy of atezolizumab in patients with LM from the second-line setting was limited, based on the individual analysis of NSCLC cohorts (P = 0.053). PD-L1 strong expression emerged as a predominant biomarker (P = 0.015) to screen atezolizumab-advantageous patients with LM. Notably, the combination of PD-L1 and LM improved the predictive power for atezolizumab therapy in both NSCLC and urothelial cancer cohorts. Exploratory translational analysis revealed that strong expression of PD-L1 might have reversed the non-inflamed immune phenotype of liver metastasis, thus sensitizing these patients to immunotherapy.
Conclusion: Our study demonstrated a preferable efficacy of atezolizumab in patients with LM as first-line therapy over standard of care therapy, while sensitive patients should be selected in second-line settings. PD-L1 was demonstrated as the most effective biomarker for screening atezolizumab-advantageous patients with LM.
Keywords: PD-L1; advantageous; atezolizumab; liver metastasis; pooled analysis.
© 2021 Yin et al.
Conflict of interest statement
We declare that we have no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

