Formulation and in vitro evaluation of topical nanosponge-based gel containing butenafine for the treatment of fungal skin infection
- PMID: 34135673
- PMCID: PMC8180615
- DOI: 10.1016/j.jsps.2021.04.010
Formulation and in vitro evaluation of topical nanosponge-based gel containing butenafine for the treatment of fungal skin infection
Abstract
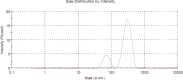
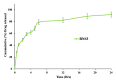
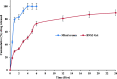
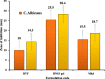
In the current study, four formulae (BNS1-BNS4) of butenafine (BTF) loaded nanosponges (NS) were fabricated by solvent emulsification technology, using different concentration of ethyl cellulose (EC) and polyvinyl alcohol (PVA) as a rate retarding polymer and surfactant, respectively. Prepared NS were characterized for particle size (PS), polydispersity index (PDI), zeta potential (ZP), entrapment efficiency (EE) and drug loading (DL). Nanocarrier BNS3 was optimized based on the particle characterizations and drug encapsulation. It was further evaluated for physicochemical characterizations; FTIR, DSC, XRD and SEM. Selected NS BNS3 composed of BTF (100 mg), EC (200 mg) and 0.3% of PVA showed, PS (543 ± 0.67 nm), PDI (0.330 ± 0.02), ZP (-33.8 ± 0.89 mV), %EE (71.3 ± 0.34%) and %DL (22.8 ± 0.67%), respectively. Fabricated NS also revealed; polymer-drug compatibility, drug-encapsulation, non-crystalline state of the drug in the spherical NS as per the physicochemical evaluations. Optimized NS (BNS3) with equivalent amount of (1%, w/w or w/v) BTF was incorporated into the (1%, w/w or w/v) carbopol gel. BTF loaded NS based gel was then evaluated for viscosity, spreadability, flux, drug diffusion, antifungal, stability and skin irritation studies. BNS3 based topical gels exhibited a flux rate of 0.18 (mg/cm2.h), drug diffusion of 89.90 ± 0.87% in 24 h with Higuchi model following anomalous non-Fickian drug release. The BNS3 based-gel could be effective against pathogenic fungal strains.
Keywords: Antifungal study; Flux; In-vitro diffusion; Nanosponges; Topical gel.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Ahmed M.M., Anwer M.K., Fatima F., Iqbal M., Ezzeldin E., Alalaiwe A., Aldawsari M.F. Development of ethylcellulose based nanosponges of apremilast: In vitro and in vivo pharmacokinetic evaluation. Lat. Am. J. Pharm. 2020;39:1292–1299.
-
- Ahmed, Mohammad Muqtader, Fatima, F., Mohammed, A.B., 2020b. Olive Oil Based Organogels for Effective Topical Delivery of Fluconazole : In-vitro Antifungal Study 32, 29–36. https://doi.org/10.9734/JPRI/2020/v32i2530821.
-
- Anwer M.K., Al-Mansoor M.A., Jamil S., Al-Shdefat R., Ansari M.N., Shakeel F. Development and evaluation of PLGA polymer based nanoparticles of quercetin. Int. J. Biol. Macromol. 2016;92:213–219. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

