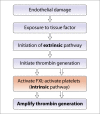
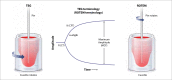
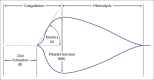
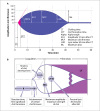
Background and Evaluation of Hypercoagulability
Conflict of interest statement
In the past 2 years, Dr Gish has received grants/research support from Gilead. He has performed as a consultant and/or advisor (in the last 2 years) to Abbott, AbbVie, Access Biologicals, Antios, Arrowhead, Bayer AG, Bristol-Myers Squibb Company, Dova, Dynavax, Eiger, Eisai, Enyo, eStudySite, Forty-Seven Inc, Genentech, Genlantis, Gerson Lehrman Group, Gilead Sciences, HepaTx, HepQuant, Intercept, Janssen, Helios, Lilly, Merck, Salix, Shionogi, and Viking Therapeutics. He is currently active on the scientific or clinical advisory boards of Abbott, AbbVie, Merck, Arrowhead, Bayer, Dova Pharmaceuticals, Eiger, Enyo, HepQuant, Intercept, and Janssen. He is a member of the Clinical Trials Alliance of Topography Health. He is the Chair of the Clinical Advisory Board of Prodigy. He is an advisory consultant for the diagnostic companies BioCollections, Fujifilm/Wako, and Quest. He is a member of the Data Safety Monitoring Board of Arrowhead. Dr Regenstein has no real or apparent conflicts of interest to report.
Figures

References
-
- Spaggiari M, Di Benedetto F, Masetti M et al. The impact of inherited thrombophilia on liver transplantation. Transplantation. 2009;87(7):1103–1104. - PubMed
-
- Abdel-Razik A, Mousa N, Elhelaly R, Tawfik A. De-novo portal vein thrombosis in liver cirrhosis: risk factors and correlation with the Model for End-stage Liver Disease scoring system. Eur J Gastroenterol Hepatol. 2015;27(5):585–592. - PubMed
-
- Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71(19):2162–2175. - PubMed
-
- Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther. 2010;31(3):366–374. - PubMed
LinkOut - more resources
Full Text Sources
