Towards a Bio-Inspired Real-Time Neuromorphic Cerebellum
- PMID: 34135732
- PMCID: PMC8202688
- DOI: 10.3389/fncel.2021.622870
Towards a Bio-Inspired Real-Time Neuromorphic Cerebellum
Abstract
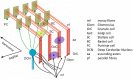
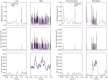
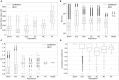
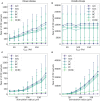
This work presents the first simulation of a large-scale, bio-physically constrained cerebellum model performed on neuromorphic hardware. A model containing 97,000 neurons and 4.2 million synapses is simulated on the SpiNNaker neuromorphic system. Results are validated against a baseline simulation of the same model executed with NEST, a popular spiking neural network simulator using generic computational resources and double precision floating point arithmetic. Individual cell and network-level spiking activity is validated in terms of average spike rates, relative lead or lag of spike times, and membrane potential dynamics of individual neurons, and SpiNNaker is shown to produce results in agreement with NEST. Once validated, the model is used to investigate how to accelerate the simulation speed of the network on the SpiNNaker system, with the future goal of creating a real-time neuromorphic cerebellum. Through detailed communication profiling, peak network activity is identified as one of the main challenges for simulation speed-up. Propagation of spiking activity through the network is measured, and will inform the future development of accelerated execution strategies for cerebellum models on neuromorphic hardware. The large ratio of granule cells to other cell types in the model results in high levels of activity converging onto few cells, with those cells having relatively larger time costs associated with the processing of communication. Organizing cells on SpiNNaker in accordance with their spatial position is shown to reduce the peak communication load by 41%. It is hoped that these insights, together with alternative parallelization strategies, will pave the way for real-time execution of large-scale, bio-physically constrained cerebellum models on SpiNNaker. This in turn will enable exploration of cerebellum-inspired controllers for neurorobotic applications, and execution of extended duration simulations over timescales that would currently be prohibitive using conventional computational platforms.
Keywords: SpiNNaker; cerebellum model; communication profiling; large scale simulation; neuromorphic computing; spiking neural network.
Copyright © 2021 Bogdan, Marcinnò, Casellato, Casali, Rowley, Hopkins, Leporati, D'Angelo and Rhodes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Albada S. J. V., Rowley A. G., Senk J., Hopkins M., Schmidt M., Stokes A. B., et al. . (2018). Performance comparison of the digital neuromorphic hardware SpiNNaker and the neural network simulation software NEST for a full-scale cortical microcircuit model. Front. Neurosci. 12:291. 10.3389/fnins.2018.00291 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

