Transcriptome Profiling Analysis of the Testis After Eyestalk Ablation for Selection of the Candidate Genes Involved in the Male Sexual Development in Macrobrachium nipponense
- PMID: 34135943
- PMCID: PMC8202825
- DOI: 10.3389/fgene.2021.675928
Transcriptome Profiling Analysis of the Testis After Eyestalk Ablation for Selection of the Candidate Genes Involved in the Male Sexual Development in Macrobrachium nipponense
Abstract
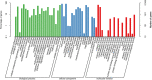
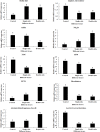
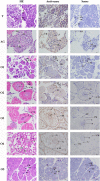
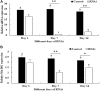
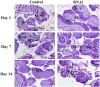
The eyestalk of crustacean species secretes many hormones, affecting the process of reproduction, molting, metabolism of glucose, and other functions in crustaceans. In this study, important metabolic pathways and candidate genes involved in the male sexual development were identified through performing the transcriptome profiling analysis of the testis after the ablation of eyestalk from Macrobrachium nipponense. The histological observations revealed that the testis development became vigorous after eyestalk ablation, indicating that the hormones secreted by the eyestalk have negative effects on the testis development in M. nipponense. Transcriptome profiling analysis revealed that 1,039, 1,226, and 3,682 differentially expressed genes (DEGs) were identified between normal prawns (CG) vs single-side eyestalk ablation prawns (SS), SS vs double-side eyestalk ablation prawns (DS), and CG vs DS, respectively, indicating that the ablation of double-side eyestalk has more significant regulatory roles on male sexual development than that of single-side ablation, which was consistent with the histological observations. Lysosome, Apoptosis, Glycolysis/Gluconeogenesis, and Insulin signaling pathway were the main enriched metabolic pathways in all of these three comparisons, and the important genes from these metabolic pathways were also selected. The qPCR verifications of 10 DEGs from these metabolic pathways were the same as those of RNA-seq. The qPCR, in situ hybridization, and RNA interference analysis of Mn-NFkBα revealed that NFkBα has a positive regulatory effect on testis development. This study provided new insights on male sexual development in M. nipponense, promoting the studies on male sexual development in other crustaceans as well.
Keywords: Macrobrachium nipponense; NFkBα; eyestalk ablation; male sexual development; testis.
Copyright © 2021 Jin, Fu, Hu, Fu, Jiang, Xiong, Qiao, Zhang, Gong and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Identification of Candidate Male-Reproduction-Related Genes from the Testis and Androgenic Gland of Macrobrachium nipponense, Regulated by PDHE1, through Transcriptome Profiling Analysis.Int J Mol Sci. 2024 Feb 5;25(3):1940. doi: 10.3390/ijms25031940. Int J Mol Sci. 2024. PMID: 38339218 Free PMC article.
-
RNA interference analysis of potential functions of cyclin A in the reproductive development of male oriental river prawns (Macrobrachium nipponense).Front Genet. 2022 Nov 17;13:1053826. doi: 10.3389/fgene.2022.1053826. eCollection 2022. Front Genet. 2022. PMID: 36467995 Free PMC article.
-
Identification of candidate genes from androgenic gland in Macrobrachium nipponense regulated by eyestalk ablation.Sci Rep. 2021 Oct 6;11(1):19855. doi: 10.1038/s41598-021-99022-4. Sci Rep. 2021. PMID: 34615913 Free PMC article.
-
Analysis of testis metabolome and transcriptome from the oriental river prawn (Macrobrachium nipponense) in response to different temperatures and illumination times.Comp Biochem Physiol Part D Genomics Proteomics. 2020 Jun;34:100662. doi: 10.1016/j.cbd.2020.100662. Epub 2020 Feb 13. Comp Biochem Physiol Part D Genomics Proteomics. 2020. PMID: 32114312
-
Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics.Gen Comp Endocrinol. 2020 Aug 1;294:113493. doi: 10.1016/j.ygcen.2020.113493. Epub 2020 Apr 24. Gen Comp Endocrinol. 2020. PMID: 32339519 Review.
Cited by
-
Identification of Male Sex-Related Genes Regulated by SDHB in Macrobrachium nipponense Based on Transcriptome Analysis after an RNAi Knockdown.Int J Mol Sci. 2023 Aug 24;24(17):13176. doi: 10.3390/ijms241713176. Int J Mol Sci. 2023. PMID: 37685979 Free PMC article.
-
PacBio single-molecule long-read sequencing provides new insights into the complexity of full-length transcripts in oriental river prawn, macrobrachium nipponense.BMC Genomics. 2023 Jun 20;24(1):340. doi: 10.1186/s12864-023-09442-x. BMC Genomics. 2023. PMID: 37340366 Free PMC article.
-
Identification of Candidate Male-Reproduction-Related Genes from the Testis and Androgenic Gland of Macrobrachium nipponense, Regulated by PDHE1, through Transcriptome Profiling Analysis.Int J Mol Sci. 2024 Feb 5;25(3):1940. doi: 10.3390/ijms25031940. Int J Mol Sci. 2024. PMID: 38339218 Free PMC article.
-
RNA interference analysis of potential functions of cyclin A in the reproductive development of male oriental river prawns (Macrobrachium nipponense).Front Genet. 2022 Nov 17;13:1053826. doi: 10.3389/fgene.2022.1053826. eCollection 2022. Front Genet. 2022. PMID: 36467995 Free PMC article.
-
RNAi Analysis of Potential Functions of Cyclin B3 in Reproduction of Male Oriental River Prawns (Macrobrachium nipponense).Animals (Basel). 2023 May 21;13(10):1703. doi: 10.3390/ani13101703. Animals (Basel). 2023. PMID: 37238135 Free PMC article.
References
-
- Almeida E. A., Petersen R. L., Andreatta E. R., Bainy A. C. (2004). Effects of captivity and eyestalk ablation on antioxidant status of shrimps (Farfantepenaeus paulensis). Aquaculture 238 523–528. 10.1016/j.aquaculture.2004.04.010 - DOI
LinkOut - more resources
Full Text Sources

