SARS-CoV-2 and the role of fomite transmission: a systematic review
- PMID: 34136133
- PMCID: PMC8176266
- DOI: 10.12688/f1000research.51590.3
SARS-CoV-2 and the role of fomite transmission: a systematic review
Abstract
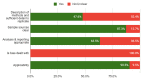
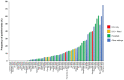
Background: SARS-CoV-2 RNA has been detected in fomites which suggests the virus could be transmitted via inanimate objects. However, there is uncertainty about the mechanistic pathway for such transmissions. Our objective was to identify, appraise and summarise the evidence from primary studies and systematic reviews assessing the role of fomites in transmission. Methods: This review is part of an Open Evidence Review on Transmission Dynamics of SARS-CoV-2. We conduct ongoing searches using WHO Covid-19 Database, LitCovid, medRxiv, and Google Scholar; assess study quality based on five criteria and report important findings on an ongoing basis. Results: We found 64 studies: 63 primary studies and one systematic review (n=35). The settings for primary studies were predominantly in hospitals (69.8%) including general wards, ICU and SARS-CoV-2 isolation wards. There were variations in the study designs including timing of sample collection, hygiene procedures, ventilation settings and cycle threshold. The overall quality of reporting was low to moderate. The frequency of positive SARS-CoV-2 tests across 51 studies (using RT-PCR) ranged from 0.5% to 75%. Cycle threshold values ranged from 20.8 to 44.1. Viral concentrations were reported in 17 studies; however, discrepancies in the methods for estimation prevented comparison. Eleven studies (17.5%) attempted viral culture, but none found a cytopathic effect. Results of the systematic review showed that healthcare settings were most frequently tested (25/35, 71.4%), but laboratories reported the highest frequency of contaminated surfaces (20.5%, 17/83). Conclusions: The majority of studies report identification of SARS-CoV-2 RNA on inanimate surfaces; however, there is a lack of evidence demonstrating the recovery of viable virus. Lack of positive viral cultures suggests that the risk of transmission of SARS-CoV-2 through fomites is low. Heterogeneity in study designs and methodology prevents comparisons of findings across studies. Standardized guidelines for conducting and reporting research on fomite transmission is warranted.
Keywords: COVID-19; Fomites; systematic review; transmission.
Copyright: © 2021 Onakpoya IJ et al.
Conflict of interest statement
Competing interests: CJH holds grant funding from the NIHR, the NIHR School of Primary Care Research, the NIHR BRC Oxford and the World Health Organization for a series of Living rapid review on the modes of transmission of SARs-CoV-2 reference WHO registration No2020/1077093. He has received financial remuneration from an asbestos case and given legal advice on mesh and hormone pregnancy tests cases. He has received expenses and fees for his media work including occasional payments from BBC Radio 4 Inside Health and The Spectator. He receives expenses for teaching EBM and is also paid for his GP work in NHS out of hours (contract Oxford Health NHS Foundation Trust). He has also received income from the publication of a series of toolkit books and for appraising treatment recommendations in non-NHS settings. He is Director of CEBM, an NIHR Senior Investigator and an advisor to Collateral Global. He is also an editor of the Cochrane Acute Respiratory Infections Group. JB is a major shareholder in the Trip Database search engine (www.tripdatabase.com) as well as being an employee. In relation to this work Trip has worked with a large number of organisations over the years, none have any links with this work. The main current projects are with AXA and Collateral Global. AP is Senior Research Fellow at the Centre for Evidence-Based Medicine and reports grant funding from NIHR School of Primary Care Research (NIHR SPCR ESWG project 390 and project 461), during the conduct of the study; and occasionally receives expenses for teaching Evidence-Based Medicine. DHE holds grant funding from the Canadian Institutes for Health Research and Li Ka Shing Institute of Virology relating to the development of Covid-19 vaccines as well as the Canadian Natural Science and Engineering Research Council concerning Covid-19 aerosol transmission. He is a recipient of World Health Organization and Province of Alberta funding which supports the provision of BSL3-based SARS-CoV-2 culture services to regional investigators. He also holds public and private sector contract funding relating to the development of poxvirus-based Covid-19 vaccines, SARS-CoV-2-inactivation technologies, and serum neutralization testing. JMC holds grants from the Canadian Institutes for Health Research on acute and primary care preparedness for COVID 19 in Alberta, Canada and was the primary local Investigator for a Staphylococcus aureus vaccine study funded by Pfizer for which all funding was provided only to the University of Calgary. He is a co investigator on a WHO funded study using integrated human factors and ethnography approaches to identify and scale innovative IPC guidance implementation supports in primary care with a focus on low resource settings and using drone aerial systems to deliver medical supplies and PPE to remote First Nations communities during the COVID 19 pandemic. He also received support from the Centers for Disease Control and Prevention (CDC) to attend an Infection Control Think Tank Meeting. He is a member of the WHO Infection Prevention and Control Research and Development Expert Group for COVID 19 and the WHO Health Emergencies Programme (WHE) Ad hoc COVID 19 IPC Guidance Development Group, both of which provide multidisciplinary advice to the WHO, for which no funding is received and from which no funding recommendations are made for any WHO contracts or grants. He is also a member of the Cochrane Acute Respiratory Infections Group. TJ was in receipt of a Cochrane Methods Innovations Fund grant to develop guidance on the use of regulatory data in Cochrane reviews (2015-018). In 2014–2016, he was a member of three advisory boards for Boehringer Ingelheim. TJ was a member of an independent data monitoring committee for a Sanofi Pasteur clinical trial on an influenza vaccine. TJ is occasionally interviewed by market research companies about phase I or II pharmaceutical products for which he receives fees (current). TJ was a member of three advisory boards for Boehringer Ingelheim (2014-16). TJ was a member of an independent data monitoring committee for a Sanofi Pasteur clinical trial on an influenza vaccine (2015-2017). TJ is a relator in a False Claims Act lawsuit on behalf of the United States that involves sales of Tamiflu for pandemic stockpiling. If resolved in the United States favor, he would be entitled to a percentage of the recovery. TJ is coholder of a Laura and John Arnold Foundation grant for development of a RIAT support centre (2017-2020) and Jean Monnet Network Grant, 2017-2020 for The Jean Monnet Health Law and Policy Network. TJ is an unpaid collaborator to the project Beyond Transparency in Pharmaceutical Research and Regulation led by Dalhousie University and funded by the Canadian Institutes of Health Research (2018-2022). TJ consulted for Illumina LLC on next generation gene sequencing (2019-2020). TJ was the consultant scientific coordinator for the HTA Medical Technology programme of the Agenzia per i Servizi Sanitari Nazionali (AGENAS) of the Italian MoH (2007-2019). TJ is Director Medical Affairs for BC Solutions, a market access company for medical devices in Europe. TJ was funded by NIHR UK and the World Health Organization (WHO) to update Cochrane review A122, Physical Interventions to interrupt the spread of respiratory viruses. TJ is funded by Oxford University to carry out a living review on the transmission epidemiology of COVID-19. Since 2020, TJ receives fees for articles published by The Spectator and other media outlets. TJ is part of a review group carrying out Living rapid literature review on the modes of transmission of SARS-CoV-2 (WHO Registration 2020/1077093-0). He is a member of the WHO COVID-19 Infection Prevention and Control Research Working Group for which he receives no funds. TJ is funded to co-author rapid reviews on the impact of Covid restrictions by the Collateral Global Organisation. He is also an editor of the Cochrane Acute Respiratory Infections Group. TJ’s competing interests are also online https://restoringtrials.org/competing-interests-tom-jefferson IJO and EAS have no interests to disclose.
Figures
References
-
- World Health Organization: WHO Coronavirus Disease (COVID-19) Dashboard. [Accessed 18/01/2021]. Reference Source
-
- World Health Organization: Transmission of SARS-CoV-2: implications for infection prevention precautions. [Accessed 16/01/2021]. Reference Source
-
- World Health Organization: Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. [Accessed 16/01/2021]. Reference Source
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous