Clinical studies of traditional Japanese herbal medicines (Kampo): Need for evidence by the modern scientific methodology
- PMID: 34136346
- PMCID: PMC8181179
- DOI: 10.1016/j.imr.2021.100722
Clinical studies of traditional Japanese herbal medicines (Kampo): Need for evidence by the modern scientific methodology
Abstract
Background: Japanese Kampo medicine is a traditional medicine with roots in ancient Chinese medicine. Because traditional physicians had been abolished in Japan, the present mainstream of Kampo treatment is that physicians who learned modern Western medicine prescribe Kampo extract products based on Western medical diagnosis. This situation is different from that in other east Asian countries, and the physicians require scientific clinical evidence.
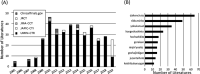
Methods: Clinical studies were searched from literature databases, clinical trial registry sites, and "Evidence Reports of Kampo Treatment (EKAT)" published by the Japan Society for Oriental Medicine.
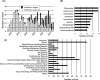
Results: At the approval of Kampo products, scientific clinical evidence was not required because they have a long-period experience as a decoction. However, in the 1990s, Kampo products became a subject for national reevaluation; double-blind and placebo-controlled clinical trials. At the time, a methodological foundation for conducting clinical assessments of Kampo medicines was established. From 2000 onwards, with the evidence-based medicine era, the field of Kampo medicine also saw many randomized controlled trials, and their evidence was collected and published as EKAT. In the 2010s, post-marketing clinical trials of Kampo products also had to be conducted in this environment due to the need for ethical and scientific assurance. Currently, there are numerous clinical trials of Kampo products being conducted with high-grade trial designs.
Conclusion: The situation of Kampo clinical studies reflects the unique history and position of Kampo medical system and Kampo products in Japan.
Keywords: Clinical study; Clinical trial registry; Kampo medicine; Randomized controlled trial; Reevaluation.
© 2021 Published by Elsevier B.V. on behalf of Korea Institute of Oriental Medicine.
Figures

References
-
- Arai I, Kawahara N. Kampo pharmaceutical products in the Japanese health-care system: Legal status and quality assurance. Tradit Kampo Med. 2019;6:3–11.
-
- Public Relations Committee, Japan Kampo Medicines Manufacturers Association Survey of Kampo medicine prescription 2011. JKMA Website. 2011 http://www.nikkankyo.org/serv/pdf/jittaichousa2011.pdf PublishedAccessed July 18, 2020. [in Japanese]
-
- Arai I. History of Japanese Kampo medicines manufacturers. Yakushigaku Zasshi (Jpn J History Pharm) 2015;50:1–6. [In Japanese] - PubMed
-
- The Federation of Pharmaceutical Manufacturers' Associations of JAPAN (FPMAJ). [The reevaluation collection]. FPMAJ website: http://www.fpmaj-saihyoka.com/. Accessed January 7, 2021. [in Japanese]
-
- Committee for Evidence-based Medicine, The Japan Society for Oriental Medicine. Evidence Reports of Kampo Treatment (Japanese version). JSOM Website. http://www.jsom.or.jp/medical/ebm/er/index.html. Accessed July 18, 2020. [in Japanese]
Publication types
LinkOut - more resources
Full Text Sources

