Early Resuscitation in Paediatric Sepsis Using Inotropes - A Randomised Controlled Pilot Study in the Emergency Department (RESPOND ED): Study Protocol and Analysis Plan
- PMID: 34136441
- PMCID: PMC8200662
- DOI: 10.3389/fped.2021.663028
Early Resuscitation in Paediatric Sepsis Using Inotropes - A Randomised Controlled Pilot Study in the Emergency Department (RESPOND ED): Study Protocol and Analysis Plan
Abstract
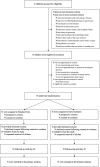
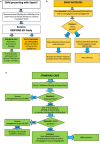
Introduction: Septic shock in children still carries substantial mortality and morbidity. While resuscitation with 40-60 mL/kg intravenous fluid boluses remains a cornerstone of initial resuscitation, an increasing body of evidence indicates potential for harm related to high volume fluid administration. We hypothesize that a protocol on early use of inotropes in children with septic shock is feasible and will lead to less fluid bolus use compared to standard fluid resuscitation. Here, we describe the protocol of the Early Resuscitation in Paediatric Sepsis Using Inotropes - A Randomised Controlled Pilot Study in the Emergency Department (RESPOND ED). Methods and analysis: The RESPOND ED study is an open label randomised controlled, two arm, multicentre pilot study conducted at four specialised paediatric Emergency Departments. Forty children aged between 28 days and 18 years treated for presumed septic shock will be randomized in a 1:1 ratio to early inotropes vs. standard fluid resuscitation. Early inotrope treatment is defined as the commencement of a continuous intravenous adrenaline infusion after 20 mL/kg fluid bolus resuscitation. Standard fluid resuscitation is defined as delivery of 40 to 60 mL/kg fluid bolus resuscitation prior to commencement of inotropes. In addition to feasibility outcomes, survival free of organ dysfunction censored at 28 days will be assessed as the main clinical outcome. The study cohort will be followed up at 28 days, and at 6 months post enrolment to assess quality of life and functional status. Biobanking nested in the study cohort will be performed to enable ancillary biomarker studies. Ethics and dissemination: The trial has ethical clearance (Children's Health Queensland, Brisbane, HREC/18/QCHQ/49168) and is registered in the Australian New Zealand Clinical Trials Registry (ACTRN12619000828123). Enrolment commenced on July 21st, 2019. The primary manuscript will be submitted for publication in a peer-reviewed journal. Trial Registration: Australian and New Zealand Clinical Trials Registry, ACTRN12619000828123.
Keywords: fluid; inotrope; pediatric; resuscitation; sepsis; septic shock (MeSH); trial.
Copyright © 2021 Harley, George, King, Phillips, Keijzers, Long, Gibbons, Bellomo and Schlapbach.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Resuscitation With Early Adrenaline Infusion for Children With Septic Shock: A Randomized Pilot Trial.Pediatr Crit Care Med. 2024 Feb 1;25(2):106-117. doi: 10.1097/PCC.0000000000003351. Epub 2024 Jan 19. Pediatr Crit Care Med. 2024. PMID: 38240535 Free PMC article. Clinical Trial.
-
Resuscitation in Paediatric Sepsis Using Metabolic Resuscitation-A Randomized Controlled Pilot Study in the Paediatric Intensive Care Unit (RESPOND PICU): Study Protocol and Analysis Plan.Front Pediatr. 2021 Apr 30;9:663435. doi: 10.3389/fped.2021.663435. eCollection 2021. Front Pediatr. 2021. PMID: 34041208 Free PMC article.
-
Resuscitation in Paediatric Septic Shock Using Vitamin C and Hydrocortisone (RESPOND): The RESPOND Randomized Controlled Trial Protocol.Pediatr Crit Care Med. 2025 Mar 1;26(3):e374-e385. doi: 10.1097/PCC.0000000000003674. Epub 2024 Dec 26. Pediatr Crit Care Med. 2025. PMID: 39724024 Free PMC article.
-
Is There an Optimum Duration of Fluid Bolus in Pediatric Septic Shock? A Critical Appraisal of "Fluid Bolus Over 15-20 Versus 5-10 Minutes Each in the First Hour of Resuscitation in Children With Septic Shock: A Randomized Controlled Trial" by Sankar et al (Pediatr Crit Care Med 2017; 18:e435-e445).Pediatr Crit Care Med. 2018 Apr;19(4):369-371. doi: 10.1097/PCC.0000000000001459. Pediatr Crit Care Med. 2018. PMID: 29369077 Review.
-
A quality improvement project to improve early sepsis care in the emergency department.BMJ Qual Saf. 2015 Dec;24(12):787-95. doi: 10.1136/bmjqs-2014-003552. Epub 2015 Aug 6. BMJ Qual Saf. 2015. PMID: 26251506 Review.
Cited by
-
Comparison of outcomes between children ventilated in a non‑paediatric intensive care and a paediatric intensive care unit: A retrospective analysis.Afr J Thorac Crit Care Med. 2022 Sep 16;28(3):10.7196/AJTCCM.2022.v28i3.215. doi: 10.7196/AJTCCM.2022.v28i3.215. eCollection 2022. Afr J Thorac Crit Care Med. 2022. PMID: 36339110 Free PMC article.
-
Resuscitation With Vitamin C, Hydrocortisone, and Thiamin in Children With Septic Shock: A Multicenter Randomized Pilot Study.Pediatr Crit Care Med. 2024 Feb 1;25(2):159-170. doi: 10.1097/PCC.0000000000003346. Epub 2024 Jan 19. Pediatr Crit Care Med. 2024. PMID: 38240537 Free PMC article. Clinical Trial.
-
A pediatric perspective on World Sepsis Day in 2021: leveraging lessons from the pandemic to reduce the global pediatric sepsis burden?Am J Physiol Lung Cell Mol Physiol. 2021 Sep 1;321(3):L608-L613. doi: 10.1152/ajplung.00331.2021. Epub 2021 Aug 18. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34405733 Free PMC article. No abstract available.
-
Resuscitation With Early Adrenaline Infusion for Children With Septic Shock: A Randomized Pilot Trial.Pediatr Crit Care Med. 2024 Feb 1;25(2):106-117. doi: 10.1097/PCC.0000000000003351. Epub 2024 Jan 19. Pediatr Crit Care Med. 2024. PMID: 38240535 Free PMC article. Clinical Trial.
References
-
- Schlapbach LJ, Straney L, Alexander J, MacLaren G, Festa M, Schibler A, et al. . Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002-13: a multicentre retrospective cohort study. Lancet Infect Dis. (2015) 15:46–54. 10.1016/S1473-3099(14)71003-5 - DOI - PubMed
LinkOut - more resources
Full Text Sources

