Bursa-Derived Cells Show a Distinct Mechano-Response to Physiological and Pathological Loading in vitro
- PMID: 34136480
- PMCID: PMC8201779
- DOI: 10.3389/fcell.2021.657166
Bursa-Derived Cells Show a Distinct Mechano-Response to Physiological and Pathological Loading in vitro
Abstract
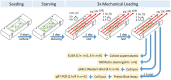
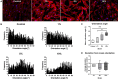
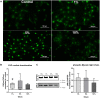
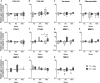
The mechano-response of highly loaded tissues such as bones or tendons is well investigated, but knowledge regarding the mechano-responsiveness of adjacent tissues such as the subacromial bursa is missing. For a better understanding of the physiological role of the bursa as a friction-reducing structure in the joint, the study aimed to analyze whether and how bursa-derived cells respond to physiological and pathological mechanical loading. This might help to overcome some of the controversies in the field regarding the role of the bursa in the development and healing of shoulder pathologies. Cells of six donors seeded on collagen-coated silicon dishes were stimulated over 3 days for 1 or 4 h with 1, 5, or 10% strain. Orientation of the actin cytoskeleton, YAP nuclear translocation, and activation of non-muscle myosin II (NMM-II) were evaluated for 4 h stimulations to get a deeper insight into mechano-transduction processes. To investigate the potential of bursa-derived cells to adapt their matrix formation and remodeling according to mechanical loading, outcome measures included cell viability, gene expression of extracellular matrix and remodeling markers, and protein secretions. The orientation angle of the actin cytoskeleton increased toward a more perpendicular direction with increased loading and lowest variations for the 5% loading group. With 10% tension load, cells were visibly stressed, indicated by loss in actin density and slightly reduced cell viability. A significantly increased YAP nuclear translocation occurred for the 1% loading group with a similar trend for the 5% group. NMM-II activation was weak for all stimulation conditions. On the gene expression level, only the expression of TIMP2 was down-regulated in the 1 h group compared to control. On the protein level, collagen type I and MMP2 increased with higher/longer straining, respectively, whereas TIMP1 secretion was reduced, resulting in an MMP/TIMP imbalance. In conclusion, this study documents for the first time a clear mechano-responsiveness in bursa-derived cells with activation of mechano-transduction pathways and thus hint to a physiological function of mechanical loading in bursa-derived cells. This study represents the basis for further investigations, which might lead to improved treatment options of subacromial bursa-related pathologies in the future.
Keywords: bursa-derived cells; matrix remodeling; mechanical stimulation; mechano-transduction; subacromial bursa.
Copyright © 2021 Klatte-Schulz, Bormann, Voss, Melzer, Schmock, Bucher, Thiele, Moroder, Haffner-Luntzer, Ignatius, Duda and Wildemann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Beard D. J., Rees J. L., Cook J. A., Rombach I., Cooper C., Merritt N., et al. (2017). Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): a multicentre, pragmatic, parallel group, placebo-controlled, three-group, randomised surgical trial. Lancet 391 329–338. 10.1016/S0140-6736(17)32457-1 - DOI - PMC - PubMed
-
- Benson R. T., McDonnell S. M., Rees J. L., Athanasou N. A., Carr A. J. (2009). The morphological and immunocytochemical features of impingement syndrome and partial-thickness rotator-cuff tear in relation to outcome after subacromial decompression. J. Bone Joint Surg. Br. 91 119–123. 10.1302/0301-620X.91B1.21058 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

