Antibody Responses to SARS-CoV-2 mRNA Vaccines Are Detectable in Saliva
- PMID: 34136730
- PMCID: PMC8201795
- DOI: 10.20411/pai.v6i1.441
Antibody Responses to SARS-CoV-2 mRNA Vaccines Are Detectable in Saliva
Abstract
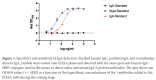
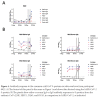
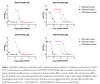
The approved Pfizer and Moderna mRNA vaccines are well known to induce serum antibody responses to the SARS-CoV-2 Spike (S)-protein. However, their abilities to elicit mucosal immune responses have not been reported. Saliva antibodies represent mucosal responses that may be relevant to how mRNA vaccines prevent oral and nasal SARS-CoV-2 transmission. Here, we describe the outcome of a cross-sectional study on a healthcare worker cohort (WELCOME-NYPH), in which we assessed whether IgM, IgG, and IgA antibodies to the S-protein and its receptor-binding domain (RBD) were present in serum and saliva samples. Anti-S-protein IgG was detected in 14/31 and 66/66 of saliva samples from uninfected participants after vaccine doses-1 and -2, respectively. IgA antibodies to the S-protein were present in 40/66 saliva samples after dose 2. Anti-S-protein IgG was present in every serum sample from recipients of 2 vaccine doses. Vaccine-induced antibodies against the RBD were also frequently present in saliva and sera. These findings may help our understanding of whether and how vaccines may impede SARS-CoV-2 transmission, including to oral cavity target cells.
Copyright © Pathogens and Immunity 2021.
Conflict of interest statement
None of the authors declares a competing interest.
Figures


Update of
-
Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva.bioRxiv [Preprint]. 2021 Mar 11:2021.03.11.434841. doi: 10.1101/2021.03.11.434841. bioRxiv. 2021. Update in: Pathog Immun. 2021 Jun 7;6(1):116-134. doi: 10.20411/pai.v6i1.441. PMID: 33758842 Free PMC article. Updated. Preprint.
References
-
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, Group CS. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–16. doi: 10.1056/NEJMoa2035389. PubMed PMID: ; PMCID: . - DOI - PMC - PubMed
-
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr., Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC, Group CCT. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–15. doi: 10.1056/NEJMoa2034577. PubMed PMID: ; PMCID: . - DOI - PMC - PubMed
-
- Huang N, Perez P, Kato T, Mikami Y, Okuda K, Gilmore RC, Conde CD, Gasmi B, Stein S, Beach M, Pe-layo E, Maldonado JO, Lafont BA, Jang SI, Nasir N, Padilla RJ, Murrah VA, Maile R, Lovell W, Wallet SM, Bowman NM, Meinig SL, Wolfgang MC, Choudhury SN, Novotny M, Aevermann BD, Scheuermann RH, Cannon G, Anderson CW, Lee RE, Marchesan JT, Bush M, Freire M, Kimple AJ, Herr DL, Rabin J, Grazioli A, Das S, French BN, Pranzatelli T, Chiorini JA, Kleiner DE, Pittaluga S, Hewitt SM, Burbelo PD, Chertow D, Consortium NC-A, Oral HCA, Craniofacial Biological N, Frank K, Lee J, Boucher RC, Teichmann SA, Warner BM, Byrd KM. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021;27(5):892–903. doi: 10.1038/s41591-021-01296-8. PubMed PMID: . - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
