Thromboembolic risk in patients with lung cancer receiving systemic therapy
- PMID: 34137029
- PMCID: PMC8283785
- DOI: 10.1111/bjh.17476
Thromboembolic risk in patients with lung cancer receiving systemic therapy
Abstract
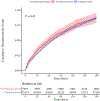
In this retrospective study, we investigated the influence of chemotherapy and immunotherapy on thromboembolic risk among United States Veterans with lung cancer during their first 6 months (180 days) following initiation of systemic therapy. Included patients received treatment with common front-line agents that were divided into four groups: chemotherapy alone, immunotherapy alone, combination of chemo- and immunotherapies, and molecularly targeted therapies (control group). The cohort experienced a 7·4% overall incidence of thrombosis, but the analysis demonstrated significantly different rates among the different groups. We explored models incorporating multiple confounding variables as well as the competing risk of death, and these results indicated that both chemo- and immunotherapies were associated with an increased incidence of thrombosis, either alone or combined, compared with the control group (7·56%, P = 2.2 × 10-16 ; 10·2%, P = 2.2 × 10-16 ; and 7·87%, P = 2.4 × 10-14 respectively vs. 4·10%). The Khorana score was found to be associated with increased risk, as were vascular disease and metastases. We found an association between risk of thrombosis and the use of anticoagulation, accounting for several confounders, including history of thrombosis. Further study is warranted to better determine the drivers of thromboembolic risk and to identify ways to mitigate this risk for patients.
Keywords: anticoagulation; chemotherapy; immunotherapy; lung cancer; thromboembolism.
© 2021 British Society for Haematology and John Wiley & Sons Ltd.
Figures




References
-
- Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost. 2017;117:219–30. - PubMed
-
- Levine MN, Gent M, Hirsh J, Arnold A, Goodyear MD, Hryniuk W, et al. The thrombogenic effect of anticancer drug therapy in women with stage II breast cancer. N Engl J Med. 1988;318:404–7. - PubMed
-
- Saphner T, Tormey DC, Gray R. Venous and arterial thrombosis in patients who received adjuvant therapy for breast cancer. J Clin Oncol. 1991;9:286–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

